
Physics, 23.09.2019 20:00 emfastback8868
Air expands adiabatically in a piston–cylinder assembly from an initial state where p1 = 100 lbf/in.2, v1 = 3.704 ft3/lb, and t1 = 1000 °r, to a final state where p2 = 30 lbf/in.2 the process is polytropic with n = 1.4. the change in specific internal energy, in btu/lb, can be expressed in terms of temperature change as (0.171)(t2 - t1). determine the final temperature, in °r. kinetic and potential energy effects can be neglected.

Answers: 1
Another question on Physics

Physics, 22.06.2019 09:30
Asap i'm in class rn a 1,000-kg car is traveling 20 m/s on a flat stretch of road. it gets to a hill and coasts uphill until it stops. how high up the hill does the car travel? givens: equation: 1/2mv2initial=mghfinal solve for h. plug & chug, label.
Answers: 2

Physics, 22.06.2019 14:30
A10nc charge sits at a point in space where the magnitude of the electric field is 1500 n/c. what will the magnitude of the field be if the 10 nc charge is replaced by a 20 nc charge? assume the system is big enough to consider the charges as small test charges.
Answers: 1

Physics, 22.06.2019 18:30
In the united states, tornadoes generally occur because of the freezing of ocean water underwater earthquakes meeting of cool and warm air masses shifting of warm and cool ocean currents
Answers: 1

Physics, 23.06.2019 05:30
Which form of pure carbon is so hard that it can be used in cutting tools?
Answers: 1
You know the right answer?
Air expands adiabatically in a piston–cylinder assembly from an initial state where p1 = 100 lbf/in....
Questions

Mathematics, 13.07.2019 19:20







Chemistry, 13.07.2019 19:20


Mathematics, 13.07.2019 19:20







Computers and Technology, 13.07.2019 19:20

Mathematics, 13.07.2019 19:20


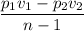
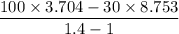
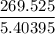 Btu/lb
Btu/lb

