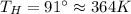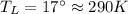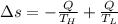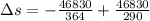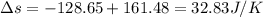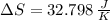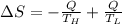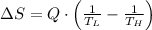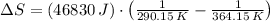Physics, 30.09.2019 23:30 noellelovebug1214
Suppose that there are two very large reservoirs of water, one at a temperature of 91.0 °c and one at a temperature of 17.0 °c. these reservoirs are brought into thermal contact long enough for 46830 j of heat to flow from the hot water to the cold water. assume that the reservoirs are large enough so that the temperatures do not change significantly. what is the total change in entropy resulting from this heat exchange between the hot water and the cold water?

Answers: 3
Another question on Physics

Physics, 21.06.2019 19:00
What term do psychologists use to designate our personal awareness of feelings, sensations, and thoughts?
Answers: 1

Physics, 22.06.2019 01:00
Avehicle has an oil leak that is causing the entire oil pan to be wet, but inspection reveals no exact source after cleaning off the oil residue. technician a says to install a fluorescent dye in the crankcase and operate the engine, then re-inspect for leaks with a special light (black light). technician b says the oil leak may be coming from a source at the top of the engine, such as a valve cover gasket. who is correct?
Answers: 1

Physics, 22.06.2019 05:30
What is a neurotransmitter involved in mood reward, addiction, and motor behaviors?
Answers: 3

You know the right answer?
Suppose that there are two very large reservoirs of water, one at a temperature of 91.0 °c and one a...
Questions

Chemistry, 19.01.2021 05:30




Social Studies, 19.01.2021 05:30


Mathematics, 19.01.2021 05:30

Mathematics, 19.01.2021 05:30

Biology, 19.01.2021 05:30

Computers and Technology, 19.01.2021 05:30



Arts, 19.01.2021 05:30

Biology, 19.01.2021 05:30




Biology, 19.01.2021 05:30

Mathematics, 19.01.2021 05:30