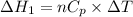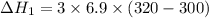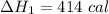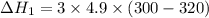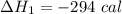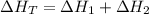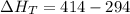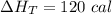Physics, 07.10.2019 18:10 maelaysiap
Three moles of an ideal gas with a molar heat capacity at constant volume of 4.9 cal/(mol∙k) and a molar heat capacity at constant pressure of 6.9 cal/(mol∙k) starts at 300 k and is heated at constant pressure to 320 k, then cooled at constant volume to its original temperature. how much heat flows into the gas during this two-step process?

Answers: 2
Another question on Physics

Physics, 21.06.2019 21:30
Aquantity of gas has a volume of 1.5 m3 and an absolute pressure of 95 kpa. when the gas is compressed to a volume of 0.5 m3, what is the new absolute pressure of the gas? (assume that there’s no change in temperature.)
Answers: 3

Physics, 22.06.2019 09:10
The diagram shows a series of volcanic island and a hot spot determine the direction of movement of the tectonic plate that for the island
Answers: 1

Physics, 22.06.2019 10:00
(a) calculate the number of electrons in a small, electrically neutral silver pin that has a mass of 10.0 g. silver has 47 electrons per atom, and its molar mass is 107.87 g/mol. (b) imagine adding electrons to the pin until the negative charge has the very large value 1.00 mc. how many electrons are added for every 109 electrons already present
Answers: 3

Physics, 22.06.2019 14:00
Estimate the change in the gibbs energy and molar gibbs energy of 1.0dm3 of octane when the pressure acting on it is increased from 1.0 atm to 100 atm. the mass density of octane is 0.703 g cm−3
Answers: 3
You know the right answer?
Three moles of an ideal gas with a molar heat capacity at constant volume of 4.9 cal/(mol∙k) and a m...
Questions





Biology, 29.09.2019 10:10

Mathematics, 29.09.2019 10:10

Business, 29.09.2019 10:10




Chemistry, 29.09.2019 10:10

Mathematics, 29.09.2019 10:10


History, 29.09.2019 10:10




History, 29.09.2019 10:20