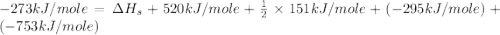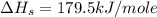Physics, 07.10.2019 18:10 dorafacegirl
Consider the following: li(s) (g) n lii(s) 222 kj. lii(s) has a lattice energy of kj/mol. the ionization energy of li(g) is 520. kj/mol, the bond en- ergy of i2(g) is 151 kj/mol, and the electron affinity of i(g) is kj/mol. use these data to determine the heat of sublimation of li(s

Answers: 1
Another question on Physics

Physics, 21.06.2019 21:00
What can be found in every skeletal muscle? a. nerves, bones, cartilage, and connective tissue b. tendons, cartilage, nerves, and blood vessels c. muscle fibers, nerves, connective tissue, and blood vessels d. tendons, nerves, blood vessels, and bones
Answers: 1

Physics, 22.06.2019 09:00
In a heat engine if 1000 j of heat enters the system the piston does 500 j of work, what is the final internal energy of the system if the initial energy was 2000 j? 1. write the equation 2.list out your known variables 3.plug the numbers into the equations 4.solve 5.write your solution statement that includes initial energy and final
Answers: 1

Physics, 22.06.2019 09:10
The air that we breath is made mostly of which gaseous molecule
Answers: 1

Physics, 22.06.2019 09:30
How would a small bar magnet be oriented when placed at position x?
Answers: 2
You know the right answer?
Consider the following: li(s) (g) n lii(s) 222 kj. lii(s) has a lattice energy of kj/mol. the...
Questions


English, 22.06.2021 05:50


Spanish, 22.06.2021 05:50

Mathematics, 22.06.2021 05:50

Mathematics, 22.06.2021 05:50


Mathematics, 22.06.2021 05:50






Mathematics, 22.06.2021 05:50

Chemistry, 22.06.2021 05:50

Mathematics, 22.06.2021 05:50



English, 22.06.2021 05:50


 = enthalpy of formation of lithium iodide
= enthalpy of formation of lithium iodide :
: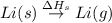
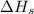 = sublimation energy of lithium
= sublimation energy of lithium 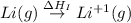
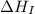 = ionization energy of lithium
= ionization energy of lithium 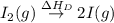
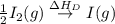
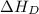 = dissociation energy of iodine
= dissociation energy of iodine 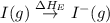
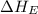 = electron affinity energy of iodine
= electron affinity energy of iodine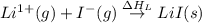
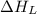 = lattice energy of lithium iodide
= lattice energy of lithium iodide