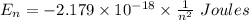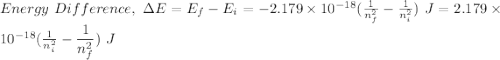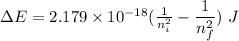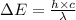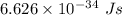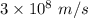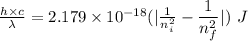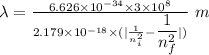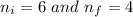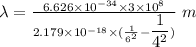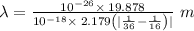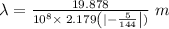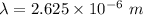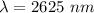Physics, 10.10.2019 05:00 justyne2004
Determine the wavelength of the photon associated with the transition in which the electron in a hydrogen atom goes from n = 6 to n = 4 a photon with a wavelength of 2694 nm is released a photon with a wavelength of 2694 nm is absorbed a photon with a wavelength of 2625 nm is absorbed a photon with a wavelength of 2533 nm is released a photon with a wavelength of 2625 nm is released

Answers: 3
Another question on Physics

Physics, 21.06.2019 14:00
How much heat is required to convert 0.3 kg of ice at 0°c to water at the same temperature
Answers: 1

Physics, 22.06.2019 12:00
Infrared radiation has wavelengths that are shorter than visible light is part of the visible light spectrum has wavelengths that are longer than visible light has the same wavelengths as ultraviolet radiation
Answers: 1

Physics, 22.06.2019 18:00
By what primary heat transfer mechanism does the sun warm the earth?
Answers: 1

Physics, 22.06.2019 18:40
Which body is in equilibrium? (1) a satellite orbiting earth in a circular orbit (2) a ball falling freely toward the surface of earth (3) a car moving with a constant speed along a straight, level road (4) a projectile at the highest point in its trajectory
Answers: 2
You know the right answer?
Determine the wavelength of the photon associated with the transition in which the electron in a hyd...
Questions




History, 25.06.2019 05:00

Biology, 25.06.2019 05:00





Mathematics, 25.06.2019 05:00

Mathematics, 25.06.2019 05:00



English, 25.06.2019 05:00

Biology, 25.06.2019 05:00

History, 25.06.2019 05:00



Spanish, 25.06.2019 05:00

Biology, 25.06.2019 05:00