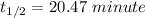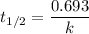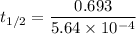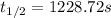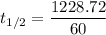Physics, 12.10.2019 03:20 shavonfriend27
At a certain temperature, the rate constant for this reaction is 5.64×10−4 s−1 . calculate the half-life of cyclopropane at this temperature.

Answers: 1
Another question on Physics

Physics, 22.06.2019 12:00
An architect plans to use solar energy to heat the next house he designs. what principle of absorption and infrared energy can be applied to the design of the new house? how could she apply to those principals?
Answers: 2

Physics, 22.06.2019 12:00
You have a resistor and a capacitor of unknown values. first, you charge the capacitor and discharge it through the resistor. by monitoring the capacitor voltage on an oscilloscope, you see that the voltage decays to half its initial value in 2.70 miss . you then use the resistor and capacitor to make a low-pass filter. what is the crossover frequency fc?
Answers: 2

Physics, 22.06.2019 18:30
4. now look at the green lines you created by connecting the three boiling point data points and the three melting point data points. for each of these lines, describe any trends you see. 5. locate the elements on your periodic table that you circled in green on your graph. what term or description would you use to identify these elements with respect to the periodic table? 7. using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers.
Answers: 2

Physics, 22.06.2019 19:30
If the area of a square has increased by a factor of 16, by how much has each side increased?
Answers: 3
You know the right answer?
At a certain temperature, the rate constant for this reaction is 5.64×10−4 s−1 . calculate the half-...
Questions

Mathematics, 31.08.2020 01:01











Mathematics, 31.08.2020 01:01



English, 31.08.2020 01:01

Chemistry, 31.08.2020 01:01

Computers and Technology, 31.08.2020 01:01