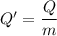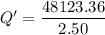Physics, 17.10.2019 20:20 awesomegrill
When 2.50 g of a certain hydrocarbon was completely combusted in a "bomb (constant-volume) calorimeter" with a heat capacity (excluding water) of 350 j/°c and which contained 2.00 liters of water (density = 1.00 g/ml and specific heat capacity = 4.184 j/°c•g), the resulting temperature change was measured to be 5.52°c. calculate the thermal energy (in kj) released per gram of hydrocarbon combusted. (1) 48.1 kj/g (2) 0.773 kj/g (3) 19.2 kj/g (4) 18.5 kj/g (5) 46.2 kj/g

Answers: 2
Another question on Physics

Physics, 21.06.2019 15:10
On a cold day, when the air temperature is 0°c, you empty your lungs and take a deep breath, filling your lungs with 4.2 l of air. if you hold your breath until the temperature of the air in your lungs reaches 37°c, what is the volume of the air in your lungs at that point, assuming the pressure does not change?
Answers: 1

Physics, 21.06.2019 22:00
What type of light does this light bulb produce most (i.e. at what wavelength does the spectrum have maximum intensity)?
Answers: 3


You know the right answer?
When 2.50 g of a certain hydrocarbon was completely combusted in a "bomb (constant-volume) calorimet...
Questions






Mathematics, 12.08.2020 08:01






History, 12.08.2020 08:01



Mathematics, 12.08.2020 08:01

Mathematics, 12.08.2020 08:01




Arts, 12.08.2020 08:01