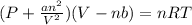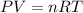choose all that apply.

Physics, 17.10.2019 22:30 romyknight
Which of the following statements is true for real gases?
choose all that apply.
a. the volume occupied by the molecules can cause an increase in pressure compared to the ideal gas.
b. the volume occupied by the molecules can cause a decrease in pressure compared to the ideal gas.
c. as molecules increase in size, deviations from ideal behavior become more apparent at relatively low pressures.
d. as attractive forces between molecules increase, deviations from ideal behavior become more apparent at relatively low temperatures.

Answers: 1
Another question on Physics

Physics, 21.06.2019 15:00
If this plastic cup is heated to its melting point, what will eventually happen to all the particles in the plastic? a. the particles will begin to move enough that they slide past each other. b. bonds will form and the liquid will become a solid. c. the spaces between the particles will get smaller. d. the particles will change state and become a crystalline solid.
Answers: 2

Physics, 22.06.2019 14:00
What is the force that opposes motion and works against the downward pull? a) friction b) gravity c) weight d) acceleration
Answers: 1

Physics, 22.06.2019 14:40
The experiment done in lab is repeated, using a ball that has unknown mass m. you plot your data in the form of f 2 versus m/l, with f in rev/s, m in kg, and l in m. your data falls close to a straight line that has slope 3.19 m/(kg · s2). use g = 9.80 m/s2 and calculate the mass m of the ball.
Answers: 1

Physics, 22.06.2019 15:20
What component of earth’s atmosphere exists entirely as a result of photosynthesis?
Answers: 2
You know the right answer?
Which of the following statements is true for real gases?
choose all that apply.
choose all that apply.
Questions







Mathematics, 02.10.2019 14:00

Biology, 02.10.2019 14:00








Advanced Placement (AP), 02.10.2019 14:00


Physics, 02.10.2019 14:00

Social Studies, 02.10.2019 14:00

Mathematics, 02.10.2019 14:00