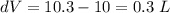Physics, 18.10.2019 17:30 marcusajns
The pressure, volume, and temperature of a mole of an ideal gas are related by the equation pv = 8.31t, where p is measured in kilopascals, v in liters, and t in kelvins. use differentials to find the approximate change in the pressure if the volume increases from 10 l to 10.3 l and the temperature decreases from 330 k to 320 k. (note whether the change is positive or negative in your answer. round your answer to two decimal places.)

Answers: 3
Another question on Physics

Physics, 22.06.2019 09:10
The air that we breath is made mostly of which gaseous molecule
Answers: 1

Physics, 22.06.2019 14:40
On a geologic map, if the contacts between sedimentary rock units form a bull’s-eye pattern of concentric circles, with the youngest unit in the center, the underlying structure is a(n)
Answers: 3

Physics, 22.06.2019 16:40
The astronauts on the space shuttle flights experienced an acceleration of 29 m/s2 (about 3 "g's") during lift-off. what upward force must the astronaut's seat apply to the astronaut in order to cause this acceleration? assume the astronaut's mass is 70 kg, and compute this force when the acceleration is near the earth's surface so their weight equals latex: mg m g .
Answers: 3

Physics, 22.06.2019 23:40
Acorporation gives out its profits as dividends paid to whom?
Answers: 1
You know the right answer?
The pressure, volume, and temperature of a mole of an ideal gas are related by the equation pv = 8.3...
Questions

History, 15.04.2021 16:00







Geography, 15.04.2021 16:00


History, 15.04.2021 16:00

Mathematics, 15.04.2021 16:00

English, 15.04.2021 16:00



Mathematics, 15.04.2021 16:00

Mathematics, 15.04.2021 16:00

Mathematics, 15.04.2021 16:00

Mathematics, 15.04.2021 16:00


 ...(I)
...(I)