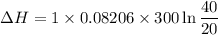One mole of a gas is placed in a closed system with a 20 l vessel initially at t = 300 k. the vessel is then isothermally expanded to 40 l. the gas follows the equation of state: p = rt/v + a/v2 where a = 240 l2 · atm/mol2 and r = 0.08206 l · atm/ mol · k. a. derive an expression relating (dh/dv)t to measurable properties. b. find dh for the gas in this process.

Answers: 2
Another question on Physics

Physics, 22.06.2019 05:00
In a stagnant pool of water, a crayfish may spend much of its time lying with one side of its carapace near the surface of the water. in this position, it will move the walking legs on that side in a rhythmic back-and-forth motion. explain the likely function of this behavior.
Answers: 1

Physics, 22.06.2019 12:00
This is for my 8th grade science class, i really don’t understand. can someone me? , lol.
Answers: 1

Physics, 22.06.2019 15:30
Two point charges are separated by a certain distance. how does the strength of the electric field produced by the first charge, at the position of the second charge, change if the second charge is doubled? a. the field does not change b. the field strength decreases by half. c. the field strength doubles d. the field strength quadruples
Answers: 1

You know the right answer?
One mole of a gas is placed in a closed system with a 20 l vessel initially at t = 300 k. the vessel...
Questions

Mathematics, 03.06.2021 02:40

Chemistry, 03.06.2021 02:40

French, 03.06.2021 02:40

Mathematics, 03.06.2021 02:40




Social Studies, 03.06.2021 02:40



Mathematics, 03.06.2021 02:40





Mathematics, 03.06.2021 02:40

Mathematics, 03.06.2021 02:40

Mathematics, 03.06.2021 02:40

Health, 03.06.2021 02:40

History, 03.06.2021 02:40

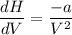
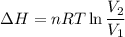 (ΔU=0)
(ΔU=0)