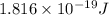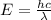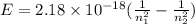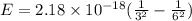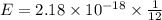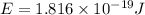An electron in a hydrogen atom undergoes a transition from the n = 3 level to the n = 6 level. to accomplish this, energy, in the form of light, must be absorbed by the hydrogen atom. calculate the energy of the light (in kj/photon) associated with this transition.

Answers: 1
Another question on Physics

Physics, 22.06.2019 21:30
What describes the formation of horizon b? a. forms at the surface b. features parent material c. undergoes the most change d. forms due to decomposed material i think the answer is c. undergoes the most change
Answers: 1

Physics, 22.06.2019 22:00
What would people living along the coast in south florida do if there was a hurricane warning? move to locations away from the water flock along coasts to watch the natural phenomenon buy instruments to predict the exact location of the hurricane measure water levels to know the exact time of the hurricane
Answers: 3

Physics, 22.06.2019 23:40
A2.50 × 105 w motor is used for 26.4 s to pull a boat straight toward shore. how far does the boat move toward shore if a force of 4.20 × 104 n is applied by the motor?
Answers: 2

Physics, 23.06.2019 01:30
At what time would the magnitude of the velocity reach 36.00 km/hr
Answers: 2
You know the right answer?
An electron in a hydrogen atom undergoes a transition from the n = 3 level to the n = 6 level. to ac...
Questions

Biology, 15.12.2020 19:50

Mathematics, 15.12.2020 19:50


Mathematics, 15.12.2020 19:50







Mathematics, 15.12.2020 19:50

Chemistry, 15.12.2020 19:50



Mathematics, 15.12.2020 19:50


Mathematics, 15.12.2020 19:50

Mathematics, 15.12.2020 19:50

Law, 15.12.2020 19:50