
A25.0-g sample of copper at 363 k is placed in i 00.0 g of water at 293 k. the copper and water quickly come to the sa me temperature by the process of heat transfer from copper to water. calculate the final temperature of the water. the molar heat capacity of copper is 24.5 j · k-1 ·mol-1 and that of water is 75.2 1 · k -1 -mol-1•

Answers: 2
Another question on Physics

Physics, 21.06.2019 19:00
Many machines- including inclined planes such as ramps- increase the strength of the force put into the machine but decrease the distance over which the force is applied. true or false
Answers: 1

Physics, 21.06.2019 22:30
According to the tide table below what time of day will the highest tide occur?
Answers: 1

Physics, 22.06.2019 15:30
Two pans of a balance are 24.1 cm apart. the fulcrum of the balance has been shifted 1.33 cm away from the center by a dishonest shopkeeper. by what percentage is the true weight of the goods being marked up by the shopkeeper? assume the balance has negligible mass. answer in units of %.
Answers: 1

Physics, 22.06.2019 17:30
How does the entropy of steam compare to the entropy of ice?
Answers: 2
You know the right answer?
A25.0-g sample of copper at 363 k is placed in i 00.0 g of water at 293 k. the copper and water quic...
Questions

Mathematics, 06.05.2021 04:00

History, 06.05.2021 04:00

Chemistry, 06.05.2021 04:00

Mathematics, 06.05.2021 04:00




Mathematics, 06.05.2021 04:00


English, 06.05.2021 04:00

Mathematics, 06.05.2021 04:00

Biology, 06.05.2021 04:00


Mathematics, 06.05.2021 04:00

English, 06.05.2021 04:00

Mathematics, 06.05.2021 04:00



English, 06.05.2021 04:00

Mathematics, 06.05.2021 04:00

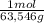 ×mass×ΔT
×mass×ΔT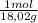 ×mass×ΔT
×mass×ΔT

