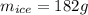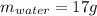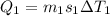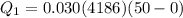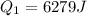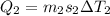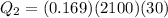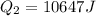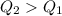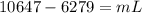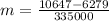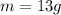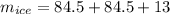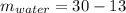Physics, 01.11.2019 01:31 makayladurham19
Two 84.5 g ice cubes are dropped into 30 g of water in a glass. if the water is initially at a temperature of 50 c and if the ice comes directly from the freezer at -300 c, what will be temperature of the drink when the ice and the water reach thermal equilibrium ? how much ice and how much water are present at thermal equilibrium ? ignore the heat capacity of the glass and heat transferred to and from the environment.

Answers: 3
Another question on Physics

Physics, 21.06.2019 18:00
Which of the following changes to a pipe would increase the conductance by a factor of 12? 1.quadrupling the length and tripling the radius.2.reducing the length by a factor of and doubling the radius.3.tripling the length and reducing the radius by a factor of .4.reducing the length but a factor of and doubling the radius.
Answers: 3


Physics, 22.06.2019 02:00
Transfer chemicals from one location to another to weather objects. a-water b-physical weathering c-chemical weathering
Answers: 2

Physics, 22.06.2019 05:30
Choose the most likely outcome of this scenario: jen decided to go bike riding without a helmet. while no one is around during her ride, she is thrown from her bike when her wheel goes into a pothole. she is not injured, but she is terrified to get back on her bike. what happens next? a. her physical health is affected even though she wasn't hurt. b. her mental and emotional health are affected because she is afraid to get back on her bike. c. her social health is affected because she is worried her friends saw the fall. d. her overall health is not affected at all by her fall.
Answers: 1
You know the right answer?
Two 84.5 g ice cubes are dropped into 30 g of water in a glass. if the water is initially at a tempe...
Questions

Mathematics, 13.10.2019 21:10

Mathematics, 13.10.2019 21:10

Chemistry, 13.10.2019 21:10


Mathematics, 13.10.2019 21:10

Mathematics, 13.10.2019 21:10

Mathematics, 13.10.2019 21:10


Mathematics, 13.10.2019 21:10


Mathematics, 13.10.2019 21:10

History, 13.10.2019 21:10

Advanced Placement (AP), 13.10.2019 21:10



Social Studies, 13.10.2019 21:10

Mathematics, 13.10.2019 21:10