Physics, 02.11.2019 04:31 ginareyes0423
An open cooking pot containing 0.5 liter of water at 20°c, 1 bar sits on a stove burner. once the burner is turned on, the water is gradually heated at a rate of 0.85 kw while pressure remains constant. after a period of time, the water starts boiling and continues to do so until all of the water has evaporated. determine a. the time required for the onset of evaporation, in s. b. the time required for all of the water to evaporate, in s, once evaporation starts.

Answers: 3
Another question on Physics

Physics, 22.06.2019 14:30
Exercise 2. find the wavelength of a photon emitted when an electron jumps from the n = 3 energy level down to the n = 2 energy level. where is this photon in the electromagnetic spectrum?
Answers: 3

Physics, 22.06.2019 17:30
You can watch static electricity in action by rubbing an inflated balloon against your hair. your hair will actually stand on its end! it does this because each hair becomes charged, and they all repel one another.
Answers: 3

Physics, 22.06.2019 20:30
Acold front traveling southeast collided with a warm front traveling northwest the following map shows the weather on monday the day the two fronts collided which of these describes the weather forecast for mississippi on tuesday and wednesday
Answers: 2

Physics, 23.06.2019 03:00
If you visited mount shasta city (elevation 900 m [3000 ft.]) and found the outside air temperature to be 27°c (81°f), what would be the air temperature at the summit of mount shasta (elevation 4200 m [14,000 ft.]) at that moment-assuming that the temperature conditions with altitude change at an average, or normal, lapse rate?
Answers: 3
You know the right answer?
An open cooking pot containing 0.5 liter of water at 20°c, 1 bar sits on a stove burner. once the bu...
Questions

English, 03.12.2020 18:00

Biology, 03.12.2020 18:00

Mathematics, 03.12.2020 18:00

Mathematics, 03.12.2020 18:00



Advanced Placement (AP), 03.12.2020 18:00



Mathematics, 03.12.2020 18:00



Health, 03.12.2020 18:00

English, 03.12.2020 18:00






Mathematics, 03.12.2020 18:10

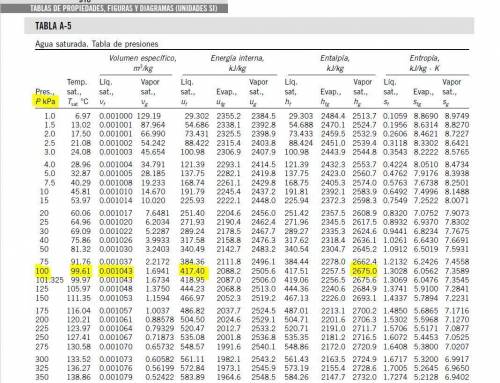
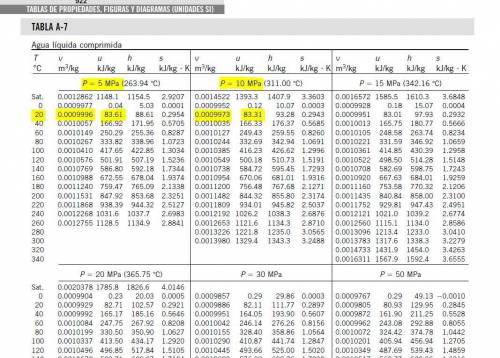
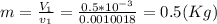 Then the liquid water is heated at a rate of 0.85KW, and its volume increase, while work is done by the system at the boundary, we can assume that the pressure remains constant throughout the entire process. At the second state the water is saturated liquid and the conditions are: P2=100KPa, T2=Tsat=99.63°C, v2=vf2=0.001043(m^3/Kg) and u2=uf2=417.36(KJ/Kg). Now we can find the work as:
Then the liquid water is heated at a rate of 0.85KW, and its volume increase, while work is done by the system at the boundary, we can assume that the pressure remains constant throughout the entire process. At the second state the water is saturated liquid and the conditions are: P2=100KPa, T2=Tsat=99.63°C, v2=vf2=0.001043(m^3/Kg) and u2=uf2=417.36(KJ/Kg). Now we can find the work as: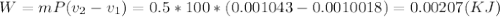 . (a) After that we need to do an energy balance for process 1-2 and get: U=Q-W or
. (a) After that we need to do an energy balance for process 1-2 and get: U=Q-W or 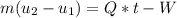 , solving for t we get the time required for the onset of evaporation:
, solving for t we get the time required for the onset of evaporation: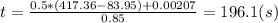 .(b) Then continue heat transfer to the cooking pot and results in phase change getting vapor at 99.63°C. At the final state or third state the mass is zero because all liquid was evaporated and the initial mass at this state is the same for the second state: 0.5 (Kg) and doing an energy balances results in:
.(b) Then continue heat transfer to the cooking pot and results in phase change getting vapor at 99.63°C. At the final state or third state the mass is zero because all liquid was evaporated and the initial mass at this state is the same for the second state: 0.5 (Kg) and doing an energy balances results in: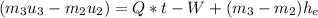 , but m3=0, now solving for t we can get the time required for all of the water to evaporate as:
, but m3=0, now solving for t we can get the time required for all of the water to evaporate as: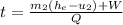 . We can get from the saturated liquid chart the enthalpy he=hge=2675.5(KJ/Kg) @P=100KPa. Now we need to calculate the work related with the volume decreases as vapor exits the control volume or process 2-3 work boundary as:
. We can get from the saturated liquid chart the enthalpy he=hge=2675.5(KJ/Kg) @P=100KPa. Now we need to calculate the work related with the volume decreases as vapor exits the control volume or process 2-3 work boundary as: 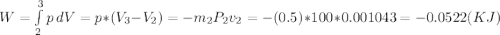 . Now replacing every value in the time equation we get:
. Now replacing every value in the time equation we get: