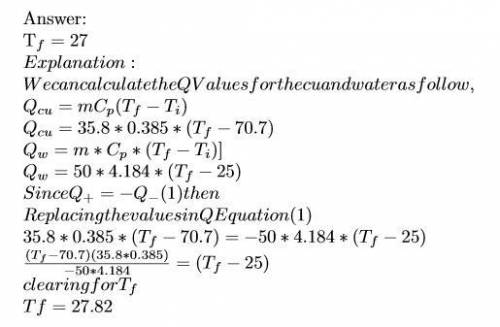
Ahot lump of 35.8 g of copper at an initial temperature of 70.7 °c is placed in 50.0 ml h2o initially at 25.0 °c and allowed to reach thermal equilibrium. what is the final temperature of the copper and water, given that the specific heat of copper is 0.385 j/(g·°c)? assume no heat is lost to surroundings.

Answers: 2
Another question on Physics

Physics, 21.06.2019 19:00
Write a question about how changing temperature affects the gas inside the sports ball
Answers: 1

Physics, 22.06.2019 00:20
Zeros that follow non-zeros numbers and are also to the right of a decimal point are significant
Answers: 2

Physics, 22.06.2019 21:00
The first law of thermodynamics states that heat added to a system is neither created nor destroyed but is as it changes into other forms of energy.
Answers: 1

Physics, 23.06.2019 02:50
You are still fascinated by the process of inkjet printing, as described in the opening storyline for this chapter. you convince your father to take you to his manufacturing facility to see the machines that print expiration dates on eggs. you strike up a conversation with the technician operating the machine. he tells you that the ink drops are created using a piezoelectric crystal, acoustic waves, and the plateau-rayleigh instability, which creates uniform drops of mass m = 1.25 ✕ 10−8 g. while you don't understand the fancy words, you do recognize mass! the technician also tells you that the drops are charged to a controllable value of q and then projected vertically downward between parallel deflecting plates at a constant terminal speed of 20.0 m/s. the plates are ℓ = 2.15 cm long and have a uniform electric field of magnitude e = 6.40 ✕ 104 n/c between them. noting your interest in the process, the technician asks you, "if the position on the egg at which the drop is to be deposited requires that its deflection at the bottom end of the plates be 0.17 mm, what is the required charge on the drop (in c)? " you quickly get to work to find the answer. (neglect the force of gravity.)
Answers: 1
You know the right answer?
Ahot lump of 35.8 g of copper at an initial temperature of 70.7 °c is placed in 50.0 ml h2o initiall...
Questions

Biology, 26.07.2019 07:20

History, 26.07.2019 07:20


Mathematics, 26.07.2019 07:20




Mathematics, 26.07.2019 07:20


History, 26.07.2019 07:20

Mathematics, 26.07.2019 07:20




History, 26.07.2019 07:20

Biology, 26.07.2019 07:20

Spanish, 26.07.2019 07:20

Mathematics, 26.07.2019 07:20

Mathematics, 26.07.2019 07:20