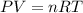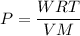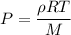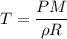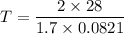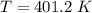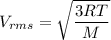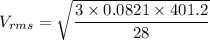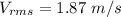Acontainer of gas molecules is at a pressure of 2 atm and has amass density of 1.7 grams per liter. all of the molecules in thecontainer are diatomic nitrogen molecules with an atomic weight of28 grams per mole. what is the typical speed of the nitrogenmolecules in the container? here we define the typical speed to bethe root-mean-square velocity (rms velocity = vrms) ofthe center of mass of the molecule

Answers: 3
Another question on Physics

Physics, 22.06.2019 00:30
Occurs when an energy source transfers heat directly to another subject space; an example would be an object becoming warm by sitting in the sunshine
Answers: 1

Physics, 22.06.2019 05:30
Which of the following are considered noble gases? a. bromine b. neon c. argon d. chlorine
Answers: 1

Physics, 22.06.2019 11:30
(1 point) match the differential equations and their vector valued function solutions. you may wish to multiply at least one solution out fully, to make sure that you know how to do it. you can get the other answers quickly by process of elimination and just multiply out one row element.
Answers: 2

Physics, 22.06.2019 12:00
Suppose a comet has an orbital period of 309.1 years around the sun. what is it’s average distance from the sun?
Answers: 1
You know the right answer?
Acontainer of gas molecules is at a pressure of 2 atm and has amass density of 1.7 grams per liter....
Questions

English, 19.12.2020 05:20

Arts, 19.12.2020 05:20


Business, 19.12.2020 05:20

English, 19.12.2020 05:20



Mathematics, 19.12.2020 05:20


English, 19.12.2020 05:20

Mathematics, 19.12.2020 05:20




Mathematics, 19.12.2020 05:20

History, 19.12.2020 05:20



Advanced Placement (AP), 19.12.2020 05:20