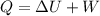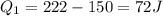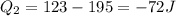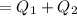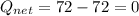Asystem undergoes a two-step process. in the first step, the internal energy of the system increases by 222 j when 150 j of work is done on the system. in the second step, the internal energy of the system increases by 123 j when 195 j of work is done on the system. for the overall process, find the heat.

Answers: 2
Another question on Physics

Physics, 21.06.2019 21:50
Which of the following is a homogenous mixture? o a. a toy box filled with toys o b. blood o c. trail mix o d. spaghetti and meatballs submit
Answers: 2

Physics, 22.06.2019 08:30
A40.0 l tank of ammonia has a pressure of 12.7 kpa. calculate the. volume of the ammonia if it’s pressure is changed to 8.4 kpa while its temperature remains constant.
Answers: 3

Physics, 22.06.2019 19:30
A47.2 g block of copper whose temperature is 480 k is placed in an insulating box with a 91.8 g block of lead whose temperature is 200 k. (a) what is the equilibrium temperature of the two-block system? (b) what is the change in the internal energy of the two-block system between the initial state and the equilibrium state? (c) what is the change in the entropy of the two-block system? the heat capacities of copper and lead are 386 j/kg·k and 128 j/kg·k, respectively.
Answers: 1

Physics, 22.06.2019 19:30
Because atoms of elements in the same group of thbecause atoms of elements in the same group of the periodic table have the same number of neutrons, they have similar properties. select the best answer from the choices provided t fe periodic table have the same number of neutrons, they have similar properties. select the best answer from the choices provided t f
Answers: 1
You know the right answer?
Asystem undergoes a two-step process. in the first step, the internal energy of the system increases...
Questions



Mathematics, 15.05.2021 05:10

Medicine, 15.05.2021 05:10


Computers and Technology, 15.05.2021 05:10


History, 15.05.2021 05:10



Mathematics, 15.05.2021 05:10


Mathematics, 15.05.2021 05:10





English, 15.05.2021 05:10


Mathematics, 15.05.2021 05:10