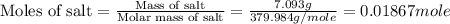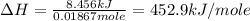Physics, 09.11.2019 00:31 PokemonCatchEmAll
Acoffee cup calorimeter is prepared, containing 100.000 g of water (specific heat capacity = 4.184 j/g k) at initial temperature 80.000 c. a salt weighing 7.093 g is quickly added. the salt has a molar mass of 379.984 g/mol. the final temperature of the solution is 61.128 c. assume no heat loss to the surroundings. assume the specific heat capacity of the solution is equal to that of pure water, and that the mass of the solution is equal to the mass of the solid plus the mass of water in the calorimeter. what is the molar heat of solution for the salt, in kj/mol? report your answer to three digits after the decimal

Answers: 1
Another question on Physics

Physics, 22.06.2019 01:00
Velocity is a description of both speed and direction, therefore it - a vector - a force arrow - the same as acceleration - a magnitude
Answers: 1

Physics, 22.06.2019 05:30
Agas expands from an initial volume of 0.040 m^3 and an initial pressure of 210 kpa to a final volume of 0.065 m^3 while its temperature is kept constant. how much work is done by the system?
Answers: 1


Physics, 22.06.2019 16:20
How does a circuit breaker protect a refrigerator? a. when the current is too high, a metal strip in the fuse melts and opens the circuit. b. when the resistance is too high , a re-settable which opens a circuit c. when the current is too high , a re-settable switch opens the circuit d. when the resistance is too high a metal strip in the fuse melts and opens the circuit
Answers: 2
You know the right answer?
Acoffee cup calorimeter is prepared, containing 100.000 g of water (specific heat capacity = 4.184 j...
Questions

English, 09.02.2021 18:20




Geography, 09.02.2021 18:20

Mathematics, 09.02.2021 18:20


English, 09.02.2021 18:20


Mathematics, 09.02.2021 18:20

Biology, 09.02.2021 18:20

Social Studies, 09.02.2021 18:20



Mathematics, 09.02.2021 18:20

Mathematics, 09.02.2021 18:20

Mathematics, 09.02.2021 18:20

English, 09.02.2021 18:20


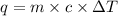
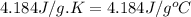
 = change in temperature =
= change in temperature = 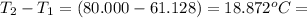
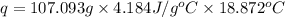
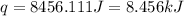
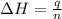
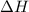 = molar heat of solution = ?
= molar heat of solution = ?