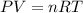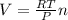Physics, 09.11.2019 04:31 randallcraig27
Avogadro's law states that: a. the volume of a fixed amount of gas is directly proportional to its temperature in kelvin at constant pressure. b. the total pressure of a mixture of gases is the simple sum of the partial pressure of all of the gaseous compounds. c. equal amounts of gases occupy the same volume at constant temperature and pressure. d. the volume of a fixed amount of gas is inversely proportional to its pressure at constant temperature. e. the rates of effusion of gases are inversely proportional to the square roots of their molar masses.

Answers: 3
Another question on Physics

Physics, 22.06.2019 15:20
What component of earth’s atmosphere exists entirely as a result of photosynthesis?
Answers: 2

Physics, 22.06.2019 19:30
Point charges q1=+2.00μc and q2=−2.00μc are placed at adjacent corners of a square for which the length of each side is 1.50 cm . point a is at the center of the square, and point b is at the empty corner closest to q2. take the electric potential to be zero at a distance far from both charges. part a what is the electric potential at point a due to q1 and q2? express your answer with the appropriate units.
Answers: 2

Physics, 22.06.2019 22:30
Suppose that three astronomical objects, 1, 2, and 3 are observed to lie on a line, and the distance from object 1 to object 3 is d. object 1 has four times the mass of object 3, and seven times the mass of object 2. find the distance between objects 1 and 2 for which the net force on object 2 is zero.
Answers: 1

You know the right answer?
Avogadro's law states that: a. the volume of a fixed amount of gas is directly proportional to its...
Questions





Mathematics, 22.02.2021 15:20


Social Studies, 22.02.2021 15:20

Social Studies, 22.02.2021 15:20

Social Studies, 22.02.2021 15:20



English, 22.02.2021 15:20

Chemistry, 22.02.2021 15:20



English, 22.02.2021 15:20

Mathematics, 22.02.2021 15:20

Physics, 22.02.2021 15:20

Mathematics, 22.02.2021 15:20

Computers and Technology, 22.02.2021 15:20