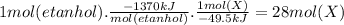Physics, 11.11.2019 22:31 adiboo2004
Assume that the complete combustion of one mole of ethanol to carbon dioxide and water liberates 1370 kj of energy (δ°′=−1370 kj/mol ). if the energy generated by the combustion of ethanol is entirely converted to the synthesis of a hypothetical compound x, calculate the number of moles of the compound that could theoretically be generated. use the value δ°′compound x=−45.9 kj/mol . round your answer to two significant figures.

Answers: 1
Another question on Physics

Physics, 21.06.2019 19:40
You carry a 7.0-kg bag of groceries 1.2 m above the ground at constant speed across a 2.7m room. how much work do you do on the bag in the process? (a) 157j (b) 0.00j (c) 185j (d) 82j
Answers: 2

Physics, 22.06.2019 07:40
Which best describes how fluids change as they travel through different portions of the convection currents? they change to solids at the outer portion of the convection currents. they change to solids at the inner portion of the convection currents. they become more dense at the outer portion of the convection currents. they become more dense at the inner portion of the convection currents
Answers: 2

Physics, 22.06.2019 08:00
The arrival of in the early days of europa’s existence could have formed its ocean. it is likely that the water experienced similar to earth. it is also possible that this water is retained beneath europa’s surface and in its atmosphere due to europa’s . 1.) a. precipitation b. water vapor c. icy debris 2.) a. gravitational compression b. biochemical cycling c. radiogenic heating 3.) a, gravity b. magnetic field c. heat energy for plato
Answers: 3

Physics, 22.06.2019 12:00
Explain why electric current cannot exist if a current doesn't have a voltage source.
Answers: 2
You know the right answer?
Assume that the complete combustion of one mole of ethanol to carbon dioxide and water liberates 137...
Questions



Chemistry, 28.01.2020 12:31

History, 28.01.2020 12:31




History, 28.01.2020 12:31

Mathematics, 28.01.2020 12:31


English, 28.01.2020 12:31

Mathematics, 28.01.2020 12:31

History, 28.01.2020 12:31



Mathematics, 28.01.2020 12:31

Chemistry, 28.01.2020 12:31


Mathematics, 28.01.2020 12:31