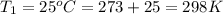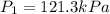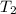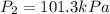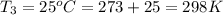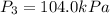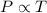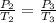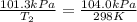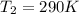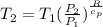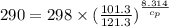Physics, 12.11.2019 02:31 jackiecroce1
Calculate the heat capacity of a gas sample from the following information: the sam- ple comes to equilibrium in a flask at 25°c and 121.3 kpa. a stopcock is opened briefly, allowing the pressure to drop to 101.3 kpa. with the stopcock closed, the flask warms, returning to 25°c, and the pressure is measured as 104.0 kpa. determine cp in j·mol−1·k−1 assuming the gas to be ideal and the expansion of the gas remaining in the flask to be reversible and adiabatic.

Answers: 3
Another question on Physics

Physics, 22.06.2019 06:00
A1,700kg car is being used to give a 1,400kg car a push start by exerting a force of 140n the impulse on the smaller car during the 30.0s of contact is +670kg*m/s. what is the impulse of the smaller car on the larger car? -814 kg*m/s 0kg *m/s -670kg*m/s -550kg*m/s
Answers: 1

Physics, 22.06.2019 12:10
The average density of the planet uranus is 1.27 103 kg/m3. the ratio of the mass of neptune to that of uranus is 1.19. the ratio of the radius of neptune to that of uranus is 0.969. find the average density of neptune.
Answers: 1

Physics, 22.06.2019 19:00
The built in flash in a compact camera is usally capable of giving correct exsposure for distance up to how many meters?
Answers: 1

Physics, 22.06.2019 19:00
On average 8 bananas make up one pound. the price is four pounds for 5.00. you must buy 25 bananas.what is your cost?
Answers: 1
You know the right answer?
Calculate the heat capacity of a gas sample from the following information: the sam- ple comes to e...
Questions

Mathematics, 16.12.2020 01:00


Mathematics, 16.12.2020 01:00

English, 16.12.2020 01:00

Mathematics, 16.12.2020 01:00



Health, 16.12.2020 01:00

Mathematics, 16.12.2020 01:00

Geography, 16.12.2020 01:00


Biology, 16.12.2020 01:00


Mathematics, 16.12.2020 01:00

Mathematics, 16.12.2020 01:00



English, 16.12.2020 01:00

Mathematics, 16.12.2020 01:00

Biology, 16.12.2020 01:00

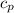 for reversible and adiabatic expansion is 55.04 J/mol.K
for reversible and adiabatic expansion is 55.04 J/mol.K