Physics, 16.11.2019 05:31 brooke0713
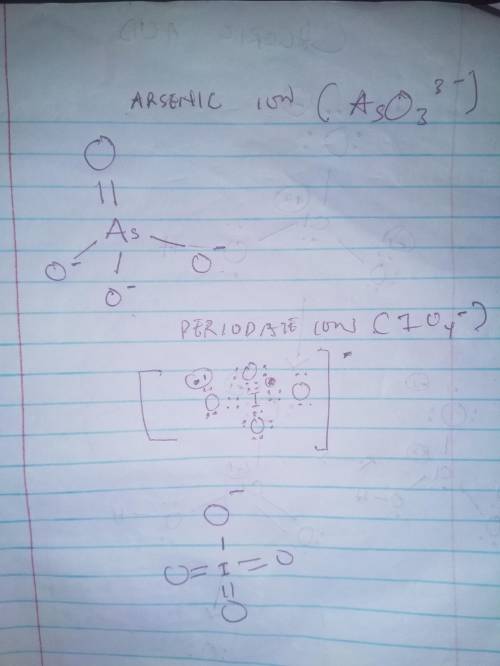
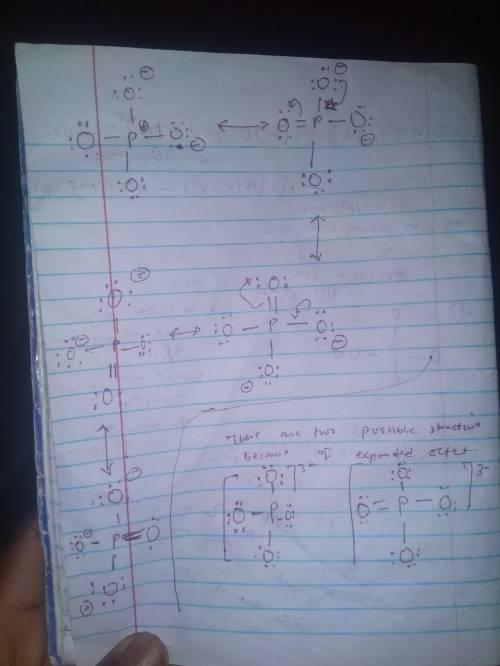
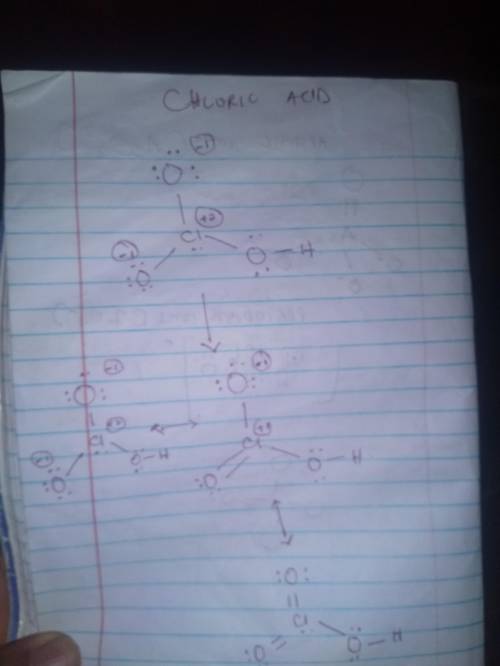
Draw the lewis structure, including typical contributions to the resonance structure (where appropriate, allow for the possibility of octet expansion, including double bonds in different positions), for (a) periodate ion; (b) hydrogen phosphate ion; (c) chloric acid; (d) arsenate ion.

Answers: 3
Another question on Physics

Physics, 21.06.2019 23:30
Around faucet handle is part of a(n) a. lever b. screw c. inclined plane d. wheel and axle
Answers: 1

Physics, 22.06.2019 11:30
Why is the energy that results from a roller coaster's position at the top of a hill referred to as potential energy?
Answers: 1

Physics, 22.06.2019 14:50
Nitrogen (n2) undergoes an internally reversible process from 6 bar, 247°c during which pν1.2 = constant. the initial volume is 0.1 m3 and the work for the process is 121.14 kj. assuming ideal gas behavior, and neglecting kinetic and potential energy effects, determine heat transfer, in kj, and the entropy change, in kj/s. show the process on a t-s diagram.
Answers: 2

Physics, 22.06.2019 15:20
Your science teacher brings in a speaker to talk to your class about climate change. during the session, students ask a few questions. which questions are related to the current evidence on climate change
Answers: 3
You know the right answer?
Draw the lewis structure, including typical contributions to the resonance structure (where appropri...
Questions


Mathematics, 09.12.2020 19:10

Advanced Placement (AP), 09.12.2020 19:10



Chemistry, 09.12.2020 19:10



Mathematics, 09.12.2020 19:10

Biology, 09.12.2020 19:10



Mathematics, 09.12.2020 19:10

Mathematics, 09.12.2020 19:10

Computers and Technology, 09.12.2020 19:10





Mathematics, 09.12.2020 19:10