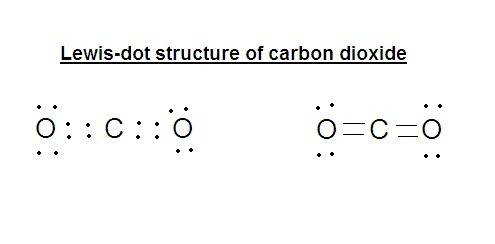
An atom of carbon (c) forms covalent bonds with two atoms of oxygen (o) to form carbon dioxide. how are the valence electrons of these atoms rearranged to form the bonds?
a. electrons are transferred from the carbon atom to the oxygen atoms.
b. electrons are transferred from the oxygen atoms to the carbon atoms.
c. many valence electrons are shared between the atoms.
d. a few valence electrons are shared between the atoms

Answers: 3
Another question on Physics

Physics, 22.06.2019 18:30
Asmall 12.00g plastic ball is suspended by a string in a uniform, horizontal electric field with a magnitude of 10^3 n/c. if the ball is in equilibrium when the string makes a 30 ° angle with the vertical, what is the net charge on the ball?
Answers: 1

Physics, 22.06.2019 18:30
Examples of states of consciousness include a. daydreaming b. dreaming during sleep c. hypnosis d. all of the above
Answers: 2

Physics, 22.06.2019 22:00
On mars a rock falls an unknown vertical distance from a resting position and lands in a crater. if it takes the rock 2.5 seconds to fall, how high is the cliff the rock fell from? mars' surface gravity is 3.8 m/s2.
Answers: 2

Physics, 22.06.2019 22:30
Isobars are lines of constant or equal pressure on a weather map. they can be used to find areas of low or high pressure over a broad area, and they can tell us how intense the system may be. on weather maps, you may have noticed areas that have a large l or h over a region with lines circling around them. the l stands for low pressure and h stands for high pressure. the lines circling them are isobars. check out the pressure system and isobars over georgia and florida. what is the present weather in this area ? a) clear and sunny b) cold and cloudy c) changing weather d) lots of precipitation
Answers: 3
You know the right answer?
An atom of carbon (c) forms covalent bonds with two atoms of oxygen (o) to form carbon dioxide. how...
Questions

English, 04.07.2021 21:50


Biology, 04.07.2021 22:00



Mathematics, 04.07.2021 22:00

Mathematics, 04.07.2021 22:00


Social Studies, 04.07.2021 22:00


Physics, 04.07.2021 22:00


Mathematics, 04.07.2021 22:00


Mathematics, 04.07.2021 22:00


Mathematics, 04.07.2021 22:00

English, 04.07.2021 22:00

Mathematics, 04.07.2021 22:00

Physics, 04.07.2021 22:00