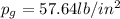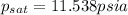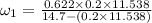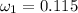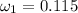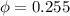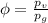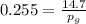Physics, 19.11.2019 06:31 fqjenfiq6959
Amixture of nitrogen and water vapor at 200of, 1 atm has the molar analysis of 80% n2, 20% water vapor. a) if the mixture is cooled at constant pressure, determine the temperature, in of, at which water vapor begins to condense. b) if the mixture is compressed at constant temperature, determine the pressure, in atm, at which water vapor begins to condense.

Answers: 3
Another question on Physics

Physics, 21.06.2019 13:50
If jill was traveling at 300 miles in 4 hours due south what was his velocity in miles per minute a) 75 miles per hour due south b) .02 miles per second due south c) .5 miles per second due south d) 1 mile per hour due south
Answers: 2

Physics, 21.06.2019 19:10
Athin, square metal plate measures 14 cm on each side and has emissivity of 0.60. the plate is heated to a temperature of 745°c. what is the rate at which the plate radiates energy ? the stefan-boltzmann constant is 5.67 × 10-8 w/(m2 ? k4). remember that the plate will radiate energy from both its top and bottom surfaces.
Answers: 1

Physics, 22.06.2019 06:40
Determine the change in width a, height b, thickness t when a plate is subjected to the uniform distributed load and is made of material having modulus of elasticity e=230 gpa and poisson's ratio ν=1/3. given : a=400 mm and b= 300 mm also the uniformly distributed load in downward y direction of plate is 2 mn/m and in the positive x direction is 3 mn/m and t=20 mm
Answers: 1

You know the right answer?
Amixture of nitrogen and water vapor at 200of, 1 atm has the molar analysis of 80% n2, 20% water vap...
Questions

Advanced Placement (AP), 21.05.2021 05:30



Mathematics, 21.05.2021 05:30

Mathematics, 21.05.2021 05:30






Geography, 21.05.2021 05:30


Mathematics, 21.05.2021 05:30

Mathematics, 21.05.2021 05:30

English, 21.05.2021 05:30

Mathematics, 21.05.2021 05:30

Advanced Placement (AP), 21.05.2021 05:30

Mathematics, 21.05.2021 05:30