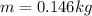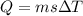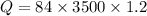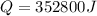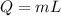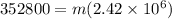Physics, 19.11.2019 20:31 datgamer13
The latent heat of vaporization of h2o at body temperature (37.0° c) is 2.42 106 j/kg. to cool the body of a 84 kg jogger (average specific heat capacity = 3500 j/(kg · c°)) by 1.2° c, how many kilograms of water in the form of sweat have to be evaporated?

Answers: 2
Another question on Physics

Physics, 21.06.2019 20:20
When many atoms are split in a chain reaction, a large explosion occurs. this is an example of what type of energy conservation
Answers: 2

Physics, 22.06.2019 20:30
Light from two different sources are incident upon the same side of a polarizing filter. light from one source is polarized with total intensity ip, while light from the other source is unpolarized with total intensity iu. as you rotate the polarizing filter through 360 degrees, the intensity of the transmitted light on the other side varies by a factor of 9. what is the relative intensity of the initial polarized source to the unpolarized source, or ip/iu?
Answers: 2

Physics, 22.06.2019 21:00
Give me a example of a decomposer. explain what would happen if decomposers were absent from a forest ecosystem
Answers: 1

Physics, 23.06.2019 04:00
A15 ohm resistor and a 25 ohm resistor are connected in parallel to a 60 volt battery. what is the power used by each device
Answers: 1
You know the right answer?
The latent heat of vaporization of h2o at body temperature (37.0° c) is 2.42 106 j/kg. to cool the b...
Questions

Mathematics, 13.10.2020 21:01

Biology, 13.10.2020 21:01

Chemistry, 13.10.2020 21:01


Mathematics, 13.10.2020 21:01


Mathematics, 13.10.2020 21:01

Physics, 13.10.2020 21:01

Mathematics, 13.10.2020 21:01


Chemistry, 13.10.2020 21:01


Mathematics, 13.10.2020 21:01

Mathematics, 13.10.2020 21:01

Biology, 13.10.2020 21:01


Health, 13.10.2020 21:01

History, 13.10.2020 21:01

History, 13.10.2020 21:01