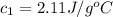Physics, 19.11.2019 23:31 keshan3000
Acube of ice at an initial temperature of -15.00°c weighing 12.5 g total is placed in 85.0 g of water at an initial temperature of 25.00°c. when thermal equilibrium is achieved, the final temperature is 22.24°c. what is the specific heat capacity of ice?

Answers: 1
Another question on Physics

Physics, 22.06.2019 07:00
World class speed skaters can skate a 3,000-m course in about 4 minutes.what is their average speed for this course? a. 12.5 m/sb. 1.33 m/sc. 1.25 m/sd. 13.3 m/s
Answers: 2

Physics, 22.06.2019 13:30
The period of a pendulum varies directly as the square root of the length of the pendulum and inversely as the square root of the acceleration due to gravity. find the period when the length is 144 cm and the acceleration due to gravity is 980 cm per second squared, if the period is 7pi seconds when the length is 289 cm and the acceleration due to gravity is 980 cm per second squared.
Answers: 2


Physics, 22.06.2019 19:30
The ability to make things happen is also called a. heat b. force c. matter d. energy
Answers: 1
You know the right answer?
Acube of ice at an initial temperature of -15.00°c weighing 12.5 g total is placed in 85.0 g of wate...
Questions

English, 20.10.2020 14:01





Mathematics, 20.10.2020 14:01





Chemistry, 20.10.2020 14:01









Biology, 20.10.2020 14:01

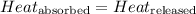
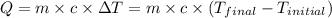
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0382/0307/09236.png) ......(1)
......(1)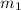 = mass of ice = 12.5 g
= mass of ice = 12.5 g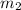 = mass of water = 85.0 g
= mass of water = 85.0 g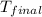 = final temperature = 22.24°C
= final temperature = 22.24°C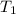 = initial temperature of ice = -15.00°C
= initial temperature of ice = -15.00°C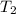 = initial temperature of water = 25.00°C
= initial temperature of water = 25.00°C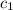 = specific heat of ice = ?
= specific heat of ice = ?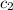 = specific heat of water = 4.186 J/g°C
= specific heat of water = 4.186 J/g°C![12.5\times c_1\times (22.24-(-15))=-[85.0\times 4.186\times (22.24-25)]](/tpl/images/0382/0307/da253.png)