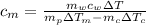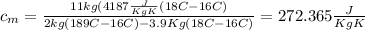Calculate the specific heat of a metal from the following data. a container made of the metal has a mass of 3.9 kg and contains 11 kg of water. a 2.0 kg piece of the metal initially at a temperature of 189°c is dropped into the water. the container and water initially have a temperature of 16.0°c, and the final temperature of the entire system is 18.0°c.

Answers: 3
Another question on Physics

Physics, 22.06.2019 02:30
Power can be defined as a. the distance over which work was done. b. how much work can be done in a given time. c. all the work in an given area. d. the energy required to do work.
Answers: 1

Physics, 22.06.2019 14:00
Acar travels in reverse. it covers 150 meters in 12 seconds what is the velocity of the car what is the speed of the car
Answers: 1


Physics, 23.06.2019 00:20
You are the coordinator for a program that is going to take place at night in a rectangular amphitheater in the mountains. you will have no access to any electricity, but you must be able to illuminate the entire grounds. you know the intensity of the light from a lantern varies inversely as the square of the distance from the lantern. suppose the intensity is 90 when the distance is 5 m. a. write an equation to model the situation. b. solve for the constant of variation. c. write the equation to model the situation using the constant () of variation. d. you have been given lanterns with 40 light intensity. use your equation to solve for the distance from the lantern. e. you need to illuminate 225 km. how many meters do you need to light? f. how many lanterns will you need?
Answers: 3
You know the right answer?
Calculate the specific heat of a metal from the following data. a container made of the metal has a...
Questions










Mathematics, 25.03.2020 05:25

History, 25.03.2020 05:25

Mathematics, 25.03.2020 05:25






History, 25.03.2020 05:25

Mathematics, 25.03.2020 05:25

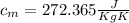
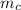 = mass of the container = 3.9 kg
= mass of the container = 3.9 kg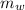 = mass of the water inside of the container= 11 kg
= mass of the water inside of the container= 11 kg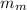 =mass of the metal= 2 kg
=mass of the metal= 2 kg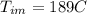 initital temperature of the metal
initital temperature of the metal initital temperature of the water
initital temperature of the water initital temperature of the container
initital temperature of the container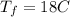 final equilibrium temperature
final equilibrium temperature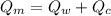
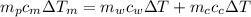
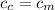
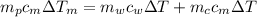
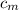 from the last expression we got:
from the last expression we got: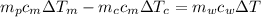
![c_m [m_p \Delta T_m -m_c \Delta T_c] =m_w c_w \Delta T](/tpl/images/0387/5562/73942.png)