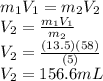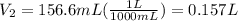Physics, 25.11.2019 21:31 KrishnaBalaram1235
Achemist must dilute 58.0 ml of 13.5 m aqueous silver nitrate (agno3)solution until the concentration falls to 5.00 m . he'll do this by adding distilled water to the solution until it reaches a certain final volume. calculate this final volume, in liters. round your answer to 3significant digits.

Answers: 1
Another question on Physics

Physics, 22.06.2019 21:30
Ineed this now one scientist feels that the changes in global climatic patterns may be good for the health of the biosphere. another scientist feels that sharp changes in climatic patterns could result in the extinction of the biosphere. which of these is the most likely reason the two scientists gave conflicting opinions on the impact of changes in the global climatic pattern? they do not have enough knowledge on the topic. they did not discuss the issue among themselves. they believe conflicting opinion strengthens science. they study different areas of science and have different scientific focuses.
Answers: 1

Physics, 23.06.2019 12:30
For this question, any non-integer answers should be entered as decimals, rounded to the hundredths place.consider this data set.the mean of the data set is , and the sample proportion of numbers less than the mean is %
Answers: 1

Physics, 23.06.2019 12:40
Remote controls rely on to carry signals and transmit information over short distances.
Answers: 1

Physics, 23.06.2019 16:00
Which tools do meteorologists use to create weather forecasts? check all that apply. a)guesses b)computer models c)weather service maps d)data from instruments e)personal observations
Answers: 1
You know the right answer?
Achemist must dilute 58.0 ml of 13.5 m aqueous silver nitrate (agno3)solution until the concentratio...
Questions

Mathematics, 14.01.2021 08:00

Mathematics, 14.01.2021 08:00


Arts, 14.01.2021 08:00




Computers and Technology, 14.01.2021 08:00

Chemistry, 14.01.2021 08:00

Social Studies, 14.01.2021 08:00



Mathematics, 14.01.2021 08:00

Biology, 14.01.2021 08:00





Physics, 14.01.2021 08:00

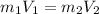
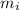 = Mass
= Mass 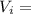 Volume
Volume 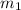 = 13.5M
= 13.5M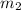 = 5M
= 5M 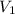 = 58.0mL
= 58.0mL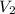 we have,
we have,