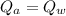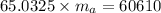Physics, 26.11.2019 20:31 shilohtito
Achunk of aluminum at 91.4°c was added to 200.0 g of water at 15.5°c. the specific heat of aluminum is 0.897 j/g°c, and the specific heat of water is 4.18 j/g°c. when the temperature stabilized, the temperature of the mixture was 18.9°c. assuming no heat was lost to the surroundings, what was the mass of aluminum added?

Answers: 3
Another question on Physics


Physics, 23.06.2019 00:30
What is the coldest temperature ever recorded in san antonio?
Answers: 1

Physics, 23.06.2019 02:30
If jason ii needs 15 kw of power at 300 volts to operate, what amperage of current must flow through the cable?
Answers: 1

Physics, 23.06.2019 06:30
If a car is traveling on the highway at a constant velocity, the force that pushes the car forward must be a. equal to the weight of the car. b. less than the force of friction exerted upon the car. c. equal to the force of friction exerted upon the car. d. greater than the weight of the car.
Answers: 1
You know the right answer?
Achunk of aluminum at 91.4°c was added to 200.0 g of water at 15.5°c. the specific heat of aluminum...
Questions

History, 05.11.2019 12:31


Physics, 05.11.2019 12:31

Social Studies, 05.11.2019 12:31

Advanced Placement (AP), 05.11.2019 12:31

English, 05.11.2019 12:31

Mathematics, 05.11.2019 12:31


Mathematics, 05.11.2019 12:31

Biology, 05.11.2019 12:31

English, 05.11.2019 12:31


Mathematics, 05.11.2019 12:31



Mathematics, 05.11.2019 12:31


Social Studies, 05.11.2019 12:31

Physics, 05.11.2019 12:31

Mathematics, 05.11.2019 12:31

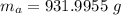
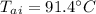 mass of water,
mass of water, 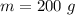 initial temperature of water,
initial temperature of water, 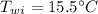 specific heat of aluminium,
specific heat of aluminium, 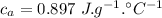 specific heat of water,
specific heat of water, 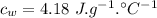 final temperature of the mixture,
final temperature of the mixture, 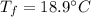
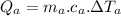
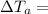 temperature difference for Al
temperature difference for Al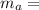 mass of aluminium
mass of aluminium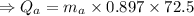
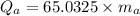 ...........................................(1)
...........................................(1)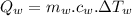
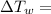 temperature difference for water
temperature difference for water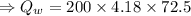
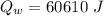 ...........................................(2)
...........................................(2)