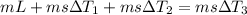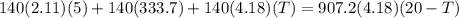Physics, 27.11.2019 00:31 maddynichole2017
In order to cool 1 ton of water at 20°c in an insulated tank, a person pours 140 kg of ice at –5°c into the water. the specific heat of water at room temperature is c = 4.18 kj/kg· °c, and the specific heat of ice at about 0°c is c = 2.11 kj/kg°c. the melting temperature and the heat of fusion of ice at 1 atm are 0°c and 333.7 kj/kg.

Answers: 1
Another question on Physics

Physics, 22.06.2019 07:30
The charge on a charged sphere is: a)concentrated at its centerb)distributed uniformly throughout its volumec)clustered on its centerd)distributed uniformly over its surface
Answers: 1

Physics, 22.06.2019 09:00
Yvette hangs a 2.4kg bird feeder in the middle of a rope tied between two trees. the feeder creates a tension of 480 n in each side of the the rope.
Answers: 1

Physics, 22.06.2019 22:00
Acar is traveling 35 mph on a smooth surface . if a balanced force is applied to the car what happens?
Answers: 3

Physics, 23.06.2019 00:00
Why does a collision with an airbag cause less damage than a collision with a steering wheel
Answers: 1
You know the right answer?
In order to cool 1 ton of water at 20°c in an insulated tank, a person pours 140 kg of ice at –5°c i...
Questions



Mathematics, 23.11.2020 21:20




Mathematics, 23.11.2020 21:20


Chemistry, 23.11.2020 21:20

Mathematics, 23.11.2020 21:20


Mathematics, 23.11.2020 21:20


Mathematics, 23.11.2020 21:20

Biology, 23.11.2020 21:20

Mathematics, 23.11.2020 21:20

Advanced Placement (AP), 23.11.2020 21:20

Social Studies, 23.11.2020 21:20


English, 23.11.2020 21:20