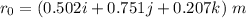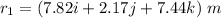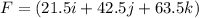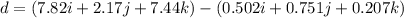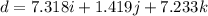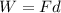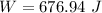Physics, 27.11.2019 03:31 mayahgrimes
Amachine carries a 33.6 kg package from an initial position of r0 = (0.502 + 0.751 + 0.207) m at t0 = 0 s to a final position of r1 = (7.82 + 2.17 + 7.44) m at t1 = 11.9 s. the constant force applied by the machine on the package is f = (21.5 + 42.5 + 63.5) n.

Answers: 1
Another question on Physics

Physics, 21.06.2019 20:50
An airplane flies eastward and always accelerates at a constant rate. at one position along its path it has a velocity of 34.3 m/s, it then flies a further distance of 40100 m and afterwards its velocity is 47.5 m/s. find the airplane\'s acceleration and calculate how much time elapses while the airplane covers those 40100 m.
Answers: 1

Physics, 22.06.2019 00:30
Glass is transparent to visibile light under normal conditions; however, at extremely high intensities, glass will absorb most of the light incident upon it. this works through a process known as multiphoton absorption. in this process, several photons are absorbed at the same time. if very intense light whose photons carry 2ev of energy is shined onto a material with a band gap of 4ev, that light can be absorbed through two-photon absorption, because two photons have the right amount of energy to bridge the band gap. what is the minimum number of photons of 800-nm light that are needed to equal or exceed the band gap of fused silica glass
Answers: 1

Physics, 22.06.2019 11:50
Two resistors r1 and r2 may be connected either in series or parallel across an ideal battery with emf ε. we desire the rate of energy dissipation of the parallel combination to be 8.75 times that of the series combination. if r1 = 105 ω, what are the (a) smaller and (b) larger of the two values of r2 that result in that dissipation rate?
Answers: 2

Physics, 22.06.2019 22:20
The starship enterprise is caught in a time warp and spock is forced to use the primitive techniques of the 20th century to determine the specific heat capacity of an unknown mineral. the 125-g sample was heated to 96.3°c and placed into a calorimeter containing 85.9 g of water at 20.0°c. the heat capacity of the calorimeter was 14.2 j/k. the final temperature in the calorimeter was 24.5°c. what is the specific heat capacity (in j/g°c) of the mineral? enter to 4 decimal places.
Answers: 1
You know the right answer?
Amachine carries a 33.6 kg package from an initial position of r0 = (0.502 + 0.751 + 0.207) m at t0...
Questions


English, 18.10.2020 01:01



Mathematics, 18.10.2020 01:01

Social Studies, 18.10.2020 01:01

Mathematics, 18.10.2020 01:01

History, 18.10.2020 01:01


History, 18.10.2020 01:01


Mathematics, 18.10.2020 01:01




Mathematics, 18.10.2020 01:01

English, 18.10.2020 01:01