
Athermally isolated system consists of a hot piece of aluminum and a cold piece of copper. the aluminum and the copper are in thermal contact. the specific heat of aluminum is more than double that of copper. which object experiences the greater amount of heat transfer during the time it takes the system to reach thermal equilibrium?

Answers: 1
Another question on Physics

Physics, 22.06.2019 05:40
Two polarizers are oriented at 40∘ to each other and plane-polarized light is incident on them. if only 16 percent of the light gets through both of them, what was the initial polarization direction of the incident light?
Answers: 2

Physics, 22.06.2019 06:00
Imagine that someone pushes one marble toward a motionless marble. would there still be action-reaction forces involved in the collision? how might the marbles’ motions be changed? ?
Answers: 1

Physics, 22.06.2019 08:30
A40.0 l tank of ammonia has a pressure of 12.7 kpa. calculate the. volume of the ammonia if it’s pressure is changed to 8.4 kpa while its temperature remains constant.
Answers: 3

Physics, 22.06.2019 14:50
Nitrogen (n2) undergoes an internally reversible process from 6 bar, 247°c during which pν1.2 = constant. the initial volume is 0.1 m3 and the work for the process is 121.14 kj. assuming ideal gas behavior, and neglecting kinetic and potential energy effects, determine heat transfer, in kj, and the entropy change, in kj/s. show the process on a t-s diagram.
Answers: 2
You know the right answer?
Athermally isolated system consists of a hot piece of aluminum and a cold piece of copper. the alumi...
Questions

Chemistry, 08.12.2020 02:20

Mathematics, 08.12.2020 02:20

Business, 08.12.2020 02:20



History, 08.12.2020 02:20

Mathematics, 08.12.2020 02:20

Mathematics, 08.12.2020 02:20


English, 08.12.2020 02:20



History, 08.12.2020 02:20



Mathematics, 08.12.2020 02:20

World Languages, 08.12.2020 02:20



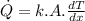
 = rate of heat transfer
= rate of heat transfer

