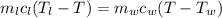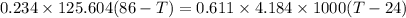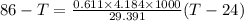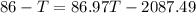Physics, 27.11.2019 06:31 fannyrivera321
A234.0 g piece of lead is heated to 86.0oc and then dropped into a calorimeter containing 611.0 g of water that initally is at 24.0oc. neglecting the heat capacity of the container, find the final equilibrium temperature (in oc) of the lead and water.

Answers: 2
Another question on Physics

Physics, 21.06.2019 17:10
An air-standard stirling cycle operates with a maximum pressure of 600 psia and a minimum pressure of 10 psia. the maximum volume of the air is 10 times the minimum volume. the temperature during the heat rejection process is 100°f. calculate the specific heat added to and rejected by this cycle, as well as the net specific work produced by the cycle. use constant specific heats at room temperature. the properties of air at room temperature are r
Answers: 2



Physics, 22.06.2019 16:40
Acapacitor is storing energy of 3 joules with a voltage of 50 volts across its terminals. a second identical capacitor of the same value is storing energy of 1 joule. what is the voltage across the terminals of the second capacitor?
Answers: 3
You know the right answer?
A234.0 g piece of lead is heated to 86.0oc and then dropped into a calorimeter containing 611.0 g of...
Questions

Mathematics, 04.01.2021 20:00


Mathematics, 04.01.2021 20:00



Computers and Technology, 04.01.2021 20:00


History, 04.01.2021 20:00

Chemistry, 04.01.2021 20:00

Arts, 04.01.2021 20:00


Mathematics, 04.01.2021 20:00

Geography, 04.01.2021 20:00


History, 04.01.2021 20:00



Mathematics, 04.01.2021 20:00

Mathematics, 04.01.2021 20:00

Computers and Technology, 04.01.2021 20:00

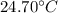
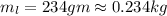
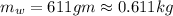
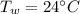
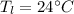
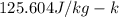 and
and 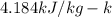 respectively
respectively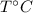 be the final temperature of the system
be the final temperature of the system