
Asample of water is heated at a constant pressure of one atmosphere. initially, the sample is ice at 260 k, and at the end the sample consists of steam at 400 k. in which of the following 5k temperature intervals would there be the greatest increase in the entropy of the sample? multiple choice
a. from 360 k to 365 k
b. from 260 k to 265 k
c. from 370 k to 375 k
d. from 395 k to 400 k
e. from 275 k to 280 k

Answers: 3
Another question on Physics

Physics, 21.06.2019 21:00
Apulley with a mechanical advantage of 5 will require you to pull times the amount of rope. a. 1/5 b. 5 c. 10 d. 15
Answers: 2


Physics, 22.06.2019 21:30
Complete the sentence to describe the law of conservation of energy. the law of conservation of energy states that energy cannot be created
Answers: 3

Physics, 22.06.2019 23:30
The photo above shows oil and vinegar in a pitcher. the top make a claim about about the density of the vinegar compared to the density of the oil summarize the evidence to support the claim and explain your reasoning
Answers: 3
You know the right answer?
Asample of water is heated at a constant pressure of one atmosphere. initially, the sample is ice at...
Questions





Biology, 22.01.2021 18:50

Health, 22.01.2021 18:50



Health, 22.01.2021 18:50



Spanish, 22.01.2021 18:50



English, 22.01.2021 18:50

Mathematics, 22.01.2021 18:50

Biology, 22.01.2021 18:50

Computers and Technology, 22.01.2021 18:50

Mathematics, 22.01.2021 18:50

Mathematics, 22.01.2021 18:50

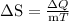 .
.
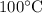 temperature of boiling water). Therefore it’s very obvious that greatest increase in entropy will occur during 370 K – 375 K.
temperature of boiling water). Therefore it’s very obvious that greatest increase in entropy will occur during 370 K – 375 K.


