Which of the following is not an assumption of the kinetic molecular theory for a gas?
a. ga...

Which of the following is not an assumption of the kinetic molecular theory for a gas?
a. gases are made up of tiny particles in constant chaotic motion.
b. gas particles are very small compared to the average distance between the particles.
c. gas particles collide with the walls of their container in elastic collisions.
d. the average velocity of the gas particles is directly proportional to the mass of the gas particles at constant temperature.
e. all of the above are assumptions of the kinetic molecular theory.

Answers: 3
Another question on Physics

Physics, 21.06.2019 17:50
In the image, the arrow is pointing to a celestial object. which attribute disqualifies the object from being a planet?
Answers: 2

Physics, 22.06.2019 07:20
If the ama of the inclined plane below is 2, calculate the ima and efficiency. ima = efficiency =
Answers: 1

Physics, 22.06.2019 08:30
Hey student studies gravity using objects that have the same mass which two objects have the greatest gravitational force acting between them a. 100kg 1.0m 100kg b. 100kg 2.0m 100kg c. 100kg 2.0m 100kg big d. 100kg big 3.0m 100kg big
Answers: 1

Physics, 22.06.2019 18:30
Which of the following is not a means to accelerating? question 4 options: a)increase speed b)remain still c)decrease speed d)change direction
Answers: 1
You know the right answer?
Questions

Physics, 07.07.2019 13:00


Mathematics, 07.07.2019 13:00

Mathematics, 07.07.2019 13:00

Biology, 07.07.2019 13:00


Biology, 07.07.2019 13:00

Social Studies, 07.07.2019 13:00

History, 07.07.2019 13:00


Mathematics, 07.07.2019 13:00


Mathematics, 07.07.2019 13:00


Mathematics, 07.07.2019 13:00


Biology, 07.07.2019 13:00

Biology, 07.07.2019 13:00

Mathematics, 07.07.2019 13:00

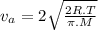
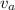 = average velocity of the gas particles
= average velocity of the gas particles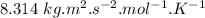 )
)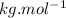 )
)

