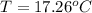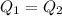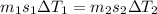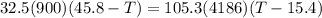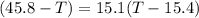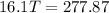Physics, 03.12.2019 01:31 Gghbhgy4809
A32.5 g cube of aluminum initially at 45.8 °c is submerged into 105.3 g of water at 15.4 °c. what is the final temperature of both substances at thermal equilibrium? (assume that the aluminum and the water are thermally isolated from everything else.)

Answers: 2
Another question on Physics

Physics, 21.06.2019 16:10
The man fires an 80 g arrow so that it is moving at 80 m/s when it hits and embeds in a 8.0 kg block resting on ice. how far will the block slide on the ice before stopping? a 7.1 n friction force opposes its motion.
Answers: 3

Physics, 22.06.2019 09:30
How would a small bar magnet be oriented when placed at position x?
Answers: 2


Physics, 23.06.2019 02:30
Which phrase describes the paleo-indians of prehistoric arkansas? a. grew cornb. lived in cliff dwellings c. crafted beautiful potteryd. hunted with stone-tipped spearswhich phrase describes native arkansas from the woodland traditiona. developed widespread agricultureb. hunted mastodon and woolly mammothc.built rectangle-shaped homesd. traded salt with other groups
Answers: 2
You know the right answer?
A32.5 g cube of aluminum initially at 45.8 °c is submerged into 105.3 g of water at 15.4 °c. what is...
Questions



Computers and Technology, 10.12.2021 02:30



Mathematics, 10.12.2021 02:30

History, 10.12.2021 02:30

Chemistry, 10.12.2021 02:30






Chemistry, 10.12.2021 02:30

Chemistry, 10.12.2021 02:30

Mathematics, 10.12.2021 02:30

Mathematics, 10.12.2021 02:30

Mathematics, 10.12.2021 02:30

Geography, 10.12.2021 02:30

Mathematics, 10.12.2021 02:30