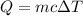Physics, 03.12.2019 18:31 gwoodbyrne
7. a block of copper of unknown mass has an initial temperature of 65.4oc. the copper is immersed in a beaker containing 95.7g of water at 22.7oc. when the two substances reach thermal equilibrium, the final temperature is 24.2oc. what is the mass of the copper block?

Answers: 2
Another question on Physics

Physics, 22.06.2019 12:30
Matter is needed to transfer thermal energy bya. conductionb. convectionc. radiation d. both a & b.
Answers: 1

Physics, 22.06.2019 13:00
The magnitude of the amount of energy released by burning a fuel source, measured in energy per unit mass, is called its fuel value. note that the fuel value is the negative of the isobaric specific heat of combustion for the fuel. if all the energy obtained from burning 1.23 pounds of butane with a fuel value of 10.85 kcal/g is used to heat 128.0 kg of water at an initial temperature of 18.3 °c, what is the final temperature? note that 1 lb = 453.6 g.
Answers: 3

Physics, 22.06.2019 14:30
70 give a real life example showing how sensory neurons work with the motor neurons
Answers: 2

Physics, 22.06.2019 14:40
During the experiment if you could triple the breakaway magnetic force with all other quantities left unchanged, what is the new value for the critical velocity if it was v0 (initial velocity), initially? (b) now if you halved the radius with all other quantities left unchanged, what is the new critical velocity if it was v0 (initial velocity), initially? (c) if during the experiment, critical velocity quadrupled with all other quantities left unchanged, what is the new breakaway force if its magnitude was initially f0,?
Answers: 1
You know the right answer?
7. a block of copper of unknown mass has an initial temperature of 65.4oc. the copper is immersed in...
Questions

Mathematics, 07.07.2019 15:30


Mathematics, 07.07.2019 15:30

History, 07.07.2019 15:30

History, 07.07.2019 15:30




Biology, 07.07.2019 15:30


Mathematics, 07.07.2019 15:30

Biology, 07.07.2019 15:30

Physics, 07.07.2019 15:30

Chemistry, 07.07.2019 15:30

Biology, 07.07.2019 15:30

Mathematics, 07.07.2019 15:30

English, 07.07.2019 15:30

Mathematics, 07.07.2019 15:30

Mathematics, 07.07.2019 15:30

 = Change in temperature
= Change in temperature