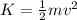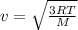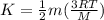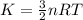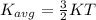Physics, 03.12.2019 19:31 guzmangisselle
Gas a has molecules with small mass. gas b has molecules with larger mass. they are at the same temperature.
how do the gases compare with respect to the average translational kinetic energy?
a)a has a larger average kinetic energy. b)b has a larger average kinetic energy. c)the gases have the same average kinetic energy.

Answers: 1
Another question on Physics

Physics, 22.06.2019 03:00
1. a net force of 100 newton’s is applied to a wagon for 5 seconds. this causes the wagon to undergo a change in momentum of
Answers: 1

Physics, 22.06.2019 03:10
Aphysical change is a change in the size, shape,, or stafe of matter true or false
Answers: 1

Physics, 22.06.2019 15:30
Identify the correct relation showing that the radius r of the orbit of a moon of a given planet can be determined from the radius r of the planet, the acceleration of gravity at the surface of the planet, and the time τ required by the moon to complete one full revolution about the planet. determine the acceleration of gravity at the surface of the planet jupiter knowing that r = 71 492 km and that t= 3.551 days and r= 670.9 × 10^3 km for its moon europa.
Answers: 2

You know the right answer?
Gas a has molecules with small mass. gas b has molecules with larger mass. they are at the same temp...
Questions




Mathematics, 23.07.2020 19:01









Chemistry, 23.07.2020 19:01


Mathematics, 23.07.2020 19:01