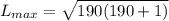Physics, 04.12.2019 00:31 elitehairnerd1964
Part a
calculate the magnitude of the maximum orbital angular momentum lmax for an electron in a hydrogen atom for states with a principal quantum number of 7.
express your answer in units of ℏ to three significant figures.
part b
calculate the magnitude of the maximum orbital angular momentum lmax for an electron in a hydrogen atom for states with a principal quantum number of 26.
express your answer in units of ℏ to three significant figures.
part c
calculate the magnitude of the maximum orbital angular momentum lmax for an electron in a hydrogen atom for states with a principal quantum number of 191.
express your answer in units of ℏ to three significant figures.

Answers: 3
Another question on Physics

Physics, 21.06.2019 18:30
Will a heavier bowling ball hit pins better than a lighter bsll
Answers: 2

Physics, 22.06.2019 02:30
Agas contained within a piston-cylinder assembly undergoes three processes in series: process 12: compression with pv= constant from 1 bar and 1 liter to 4 bar. process 23: constant pressure expansion to 1 liter. process 31: constant volume calculate the pressure and volume at each state, and sketch the processes on a p-vdiagram labeled with pressure and volume values at each numbered stat
Answers: 2

Physics, 22.06.2019 07:30
Clothes dryer uses about 7 amps of current from a 240 volt line. how much power does it use?
Answers: 1

Physics, 22.06.2019 09:00
Infrared rays have a shorter wavelength than question 11 options: x-rays. ultraviolet rays. radio waves. gamma rays.
Answers: 1
You know the right answer?
Part a
calculate the magnitude of the maximum orbital angular momentum lmax for an elec...
calculate the magnitude of the maximum orbital angular momentum lmax for an elec...
Questions



Mathematics, 23.04.2021 04:50



Mathematics, 23.04.2021 04:50


Mathematics, 23.04.2021 04:50


Mathematics, 23.04.2021 04:50

Mathematics, 23.04.2021 04:50



Mathematics, 23.04.2021 04:50

Mathematics, 23.04.2021 04:50

Engineering, 23.04.2021 04:50



Mathematics, 23.04.2021 04:50

Business, 23.04.2021 04:50

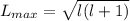 ℏ
ℏ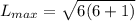 ℏ
ℏ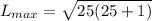 ℏ
ℏ