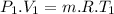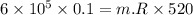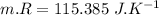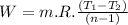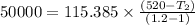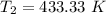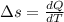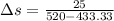Nitrogen (n2) undergoes an internally reversible process from 6 bar, 247°c during which pν1.2 = constant. the initial volume is 0.1 m3 and the work for the process is 50 kj. assuming ideal gas behavior, and neglecting kinetic and potential energy effects, determine heat transfer, in kj, and the entropy change, in kj/k.

Answers: 2
Another question on Physics

Physics, 21.06.2019 22:50
An electron and a proton have the same kinetic energy upon entering a region of constant magnetic field and their velocity vectors are perpendicular to the magnetic field. suppose the magnetic field is strong enough to allow the particles to circle in the field. note: you'll need to look up the masses for an electron and proton. 1) what is the ratio of the radii of their circular paths rp/re?
Answers: 3


Physics, 22.06.2019 14:50
Nitrogen (n2) undergoes an internally reversible process from 6 bar, 247°c during which pν1.2 = constant. the initial volume is 0.1 m3 and the work for the process is 121.14 kj. assuming ideal gas behavior, and neglecting kinetic and potential energy effects, determine heat transfer, in kj, and the entropy change, in kj/s. show the process on a t-s diagram.
Answers: 2

Physics, 22.06.2019 16:30
3. a lunar exploration vehicle was created by a research team. it weighs 3,000 kg on the earth. it needs an acceleration of 10 m/s2 on the moon. in order to have the same acceleration, what will be the net force acting on the vehicle on the earth?
Answers: 2
You know the right answer?
Nitrogen (n2) undergoes an internally reversible process from 6 bar, 247°c during which pν1.2 = cons...
Questions

English, 25.11.2021 16:30



Mathematics, 25.11.2021 16:30

Mathematics, 25.11.2021 16:30

English, 25.11.2021 16:30

Mathematics, 25.11.2021 16:30

Physics, 25.11.2021 16:30

History, 25.11.2021 16:40

Physics, 25.11.2021 16:40

Physics, 25.11.2021 16:40

Chemistry, 25.11.2021 16:40

Chemistry, 25.11.2021 16:40


Mathematics, 25.11.2021 16:40

History, 25.11.2021 16:40




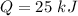
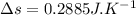
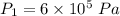 initial temperature,
initial temperature, 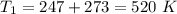 polytropic index,
polytropic index, 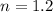 initial volume,
initial volume, 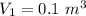 work done in the process,
work done in the process, 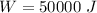
![Q=W[\frac{\gamma -n}{\gamma-1} ]](/tpl/images/0402/4036/a3071.png)
![Q=50\times[\frac{1.4-1.2}{1.4-1} ]](/tpl/images/0402/4036/3ed0d.png)