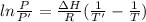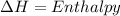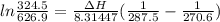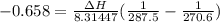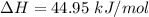The vapor pressure of the liquid hf is measured at different temperatures. the following vapor pressure data are obtained: temperature 270.6k and 287.5k, pressure 324.5 mmhg and 626.9 mmhg. calculate the enthapy of vaporization ( delta h vap ) in kj/mol for this liquid.

Answers: 2
Another question on Physics

Physics, 21.06.2019 21:00
In the 1980's, congress authorized the u.s. department of energy to build the wipp facility in the chicuahuan desert in new mexico. what is the purpose of wipp?
Answers: 2

Physics, 22.06.2019 11:30
Which of the following is the phase that results when the moon is on the opposite side of the earth from the sun? a. quarter moon b. crescent moon c. new moon d. full moon
Answers: 1

Physics, 22.06.2019 13:20
This energy transformation diagram represents the energy of a skateboarder moving along a half-pipe. as she skates toward the top of the half-pipe, her original kinetic energy is converted to potential energy and friction. how much of the energy is potential?
Answers: 3

Physics, 22.06.2019 13:30
6–43 a food department is kept at 2128c by a refrigerator in an environment at 308c. the total heat gain to the food department is estimated to be 3300 kj/h and the heat rejection in the condenser is 4800 kj/h. determine the power input to the compressor, in kw and the cop of the refrigerator.
Answers: 2
You know the right answer?
The vapor pressure of the liquid hf is measured at different temperatures. the following vapor press...
Questions


History, 28.07.2019 13:00




History, 28.07.2019 13:00





Mathematics, 28.07.2019 13:00

Biology, 28.07.2019 13:00

Biology, 28.07.2019 13:00

Chemistry, 28.07.2019 13:00




Social Studies, 28.07.2019 13:00