
Physics, 05.12.2019 03:31 lexhorton2002
The combined statement of the first and second laws for the change in enthalpy of unary single-phase system may be written: dh' = tds' +v'dp +udn use this result to write an expression for the change in enthalpy of a two-phase (alpha + beta) system. if the entropy, pressure, and total number of moles are constrained to be constant, then the criterion for equilibrium is that the enthalpy is a minimum. paraphrase the strategy used to deduce the conditions for equilibrium in an isolated system to derive them for a system constrained to constant s', p and n. what happens to the condition for mechanical equilibrium?

Answers: 2
Another question on Physics

Physics, 21.06.2019 18:00
Which surface feature of the moon is characterized by mountainous areas? terrae craters maria regolith
Answers: 1

Physics, 22.06.2019 02:00
Aweight lifter raises a 1600 n barbell to a height of 2.0 meters. how much work was done? w = fd question 6 options: 3000 joules 30 joules 320 joules 3200 joules
Answers: 2

Physics, 22.06.2019 12:30
Aboy with a mass 25 kg climbs into a small tree. he sits on a branch that is 2.o m above to the ground.what is his gravitational potential energy above the ground?
Answers: 1

You know the right answer?
The combined statement of the first and second laws for the change in enthalpy of unary single-phase...
Questions


Mathematics, 26.05.2021 16:30

Chemistry, 26.05.2021 16:30

Mathematics, 26.05.2021 16:30

Mathematics, 26.05.2021 16:30

English, 26.05.2021 16:30





Mathematics, 26.05.2021 16:30




Chemistry, 26.05.2021 16:30

Social Studies, 26.05.2021 16:30

Mathematics, 26.05.2021 16:30



Mathematics, 26.05.2021 16:30

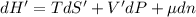
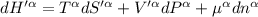
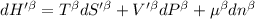
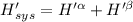
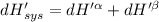
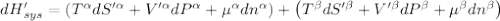
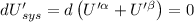
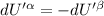
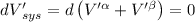
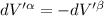
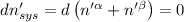
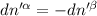
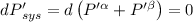
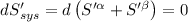
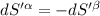
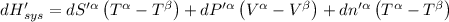
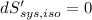
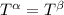 ----- for thermal equilibrium
----- for thermal equilibrium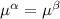 ----- for chemical equilibrium
----- for chemical equilibrium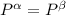 ----- for mechanical equilibrium
----- for mechanical equilibrium 

