
Physics, 05.12.2019 20:31 ryleigh8211
You place a 500 g block of an unknown substance in an insulated container filled 2 kg of water. the block has an initial temperature of 50 degrees c. the water is initially at 20 degrees c. if the equilibrium temperature of the block and water is 25 degrees c, what is the specific heat of the block? the specific heat of water is 4186 j. kg k. 3349 j, kg c 1189 j, kg c 5545 j, kg c 750 j/kg c 2080 j/kg c

Answers: 3
Another question on Physics

Physics, 22.06.2019 09:00
Infrared rays have a shorter wavelength than question 11 options: x-rays. ultraviolet rays. radio waves. gamma rays.
Answers: 1

Physics, 23.06.2019 00:00
What energy transformations occur each time the skateboarder rolls up the ramp?
Answers: 2


You know the right answer?
You place a 500 g block of an unknown substance in an insulated container filled 2 kg of water. the...
Questions

Mathematics, 12.10.2019 13:50

History, 12.10.2019 13:50


Social Studies, 12.10.2019 13:50


Computers and Technology, 12.10.2019 13:50

Mathematics, 12.10.2019 13:50



Chemistry, 12.10.2019 13:50




Mathematics, 12.10.2019 13:50

Spanish, 12.10.2019 13:50




Health, 12.10.2019 13:50

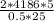 = 3348.8J/kg C≅ 3349J/kg C
= 3348.8J/kg C≅ 3349J/kg C


