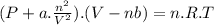Answers: 3
Another question on Physics


Physics, 22.06.2019 14:30
What was the first instrument to ever record an earthquake?
Answers: 1

Physics, 22.06.2019 19:50
An electron moves with a constant horizontal velocity of 3.0 × 106 m/s and no initial vertical velocity as it enters a deflector inside a tv tube. the electron strikes the screen after traveling 26 cm horizontally and 19 cm vertically upward with no horizontal acceleration. what is the constant vertical acceleration provided by the deflector? (the effects of gravity can be ignored.)
Answers: 2

Physics, 22.06.2019 22:00
If a hiker that weighs 600 newtons climbs a 50 meter hill, how much gravitational potential energy has the hiker gained?
Answers: 1
You know the right answer?
A9.800 mol sample of nitrogen gas is maintained in a 0.8166 l container at 301.8 k. what is the pres...
Questions



Mathematics, 19.02.2020 03:27

Mathematics, 19.02.2020 03:27

Medicine, 19.02.2020 03:28





Computers and Technology, 19.02.2020 03:28





Mathematics, 19.02.2020 03:28

History, 19.02.2020 03:28


Biology, 19.02.2020 03:28