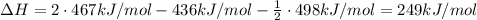What energy change is associated with the reaction to obtain one mole of h2 from one mole of water vapor? the balanced equation is 2 h2o(g) ® 2h2(g) + o2(g) and the relevant bond energies are: h — h = 436 kj/mol; h — o = 467 kj/mol; o — o = 146 kj/mol; o o = 498 kj/mol.

Answers: 1
Another question on Physics

Physics, 22.06.2019 20:00
Which is the most accurate name for the covalent compound p2o3?
Answers: 2

Physics, 23.06.2019 01:30
If the pressure in a gas is doubled while its volume is held constant. true or false
Answers: 3


Physics, 23.06.2019 04:31
Water flows through a garden hose which is attached to a nozzle. the water flows through hose with a speed of 2.19 m/s and through the nozzle with a speed of 19.8 m/s. calculate the maximum height (in m) to which water could be squirted if it emerges from the nozzle and emerges with the nozzle removed.
Answers: 1
You know the right answer?
What energy change is associated with the reaction to obtain one mole of h2 from one mole of water v...
Questions


Computers and Technology, 02.03.2021 21:00

Mathematics, 02.03.2021 21:00

Social Studies, 02.03.2021 21:00

Mathematics, 02.03.2021 21:00




Mathematics, 02.03.2021 21:00


History, 02.03.2021 21:00

History, 02.03.2021 21:00

Mathematics, 02.03.2021 21:00

History, 02.03.2021 21:00


Mathematics, 02.03.2021 21:00

English, 02.03.2021 21:00

Physics, 02.03.2021 21:00

Biology, 02.03.2021 21:00

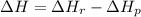
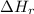 : is the bond enthalpy of reactants and
: is the bond enthalpy of reactants and 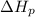 : is the bond enthalpy of products.
: is the bond enthalpy of products.