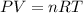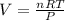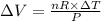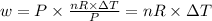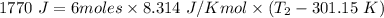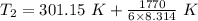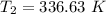Six moles of an ideal gas are in a cylinder fitted at one end with a movable piston. the initial temperature of the gas is 28.0 ∘c and the pressure is constant. part aas part of a machine design project, calculate the final temperature of the gas after it has done 1770 j .express your answer using three significant figures.

Answers: 1
Another question on Physics


Physics, 22.06.2019 14:30
Which of the following bonds would be most polar? a. c-i b. c-br c. c-cl d. c-f e. c-o
Answers: 1


Physics, 22.06.2019 17:00
Adiver named jacques observes a bubble of air rising from the bottom of a lake (where the absolute pressure is 3.50 atm) to the surface (where the pressure is 1.00 atm). the temperature at the bottom is 4.00 ∘c, and the temperature at the surface is 23.0 ∘c.what is the ratio of the volume of the bubble as it reaches the surface (vs) to its volume at the bottom (vb)? if jaques were to hold his breath the air in his lungs would be kept at a constant temperature. would it be safe for jacques to hold his breath while ascending from the bottom of the lake to the surface?
Answers: 1
You know the right answer?
Six moles of an ideal gas are in a cylinder fitted at one end with a movable piston. the initial tem...
Questions

Biology, 26.11.2019 22:31


History, 26.11.2019 22:31


Social Studies, 26.11.2019 22:31


Business, 26.11.2019 22:31


History, 26.11.2019 22:31

Mathematics, 26.11.2019 22:31



Mathematics, 26.11.2019 22:31

English, 26.11.2019 22:31

Physics, 26.11.2019 22:31

History, 26.11.2019 22:31

Mathematics, 26.11.2019 22:31



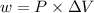
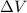 is the change in volume
is the change in volume