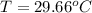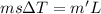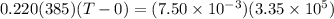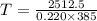Physics, 07.12.2019 00:31 herchellann302
You place an ice cube of mass 7.50×10−3kg and temperature 0.00∘c on top of a copper cube of mass 0.220 kg. all of the ice melts, and the final equilibrium temperature of the two substances is 0.00∘c. what was the initial temperature of the copper cube? assume no heat is exchanged with the surroundings.

Answers: 1
Another question on Physics

Physics, 21.06.2019 21:30
Apendulum has a mass of 1.5 kg and starts at a height of 0.4 m. if it is released from rest, how fast is it going when it reaches the lowest point of its path? acceleration due to gravity is g = 9.8 m/s2. a. 2.8 m/s b. 0 m/s c. 5.9 m/s d. 4.3 m/s
Answers: 1

Physics, 22.06.2019 04:30
Asystem containing an ideal gas at a constant pressure of 1.22×10^5 pa gains 2140 j of heat. during the process, the internal energy of the system increases by 2320 j. what is the change in volume of the gas?
Answers: 3

Physics, 22.06.2019 07:50
Determine the fraction of the magnitude of kinetic energy lost by a neutron (m1 = 1.01 u) when it collides head-on and elastically with a target particle at rest which is 21h (heavy hydrogen, m = 2.01 u).
Answers: 3

Physics, 22.06.2019 17:00
Adiver named jacques observes a bubble of air rising from the bottom of a lake (where the absolute pressure is 3.50 atm) to the surface (where the pressure is 1.00 atm). the temperature at the bottom is 4.00 ∘c, and the temperature at the surface is 23.0 ∘c.what is the ratio of the volume of the bubble as it reaches the surface (vs) to its volume at the bottom (vb)? if jaques were to hold his breath the air in his lungs would be kept at a constant temperature. would it be safe for jacques to hold his breath while ascending from the bottom of the lake to the surface?
Answers: 1
You know the right answer?
You place an ice cube of mass 7.50×10−3kg and temperature 0.00∘c on top of a copper cube of mass 0.2...
Questions

Computers and Technology, 04.04.2020 11:20






Mathematics, 04.04.2020 11:21

Mathematics, 04.04.2020 11:21