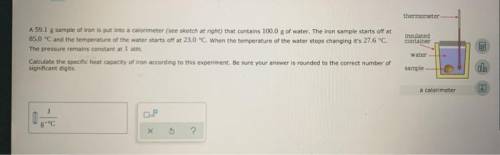
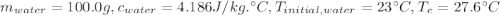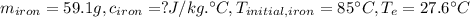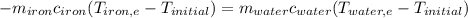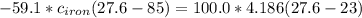Question 17 a sample of iron is put into a calorimeter (see sketch at right) that contains of water. the iron sample starts off at and the temperature of the water starts off at . when the temperature of the water stops changing it's . the pressure remains constant at . calculate the specific heat capacity of iron according to this experiment. be sure your answer is rounded to the correct number of significant digits.

Answers: 2
Another question on Physics

Physics, 22.06.2019 09:30
In a heat engine if 1000 j of heat enters the system the piston does 500 j of work, what is the final internal energy of the system if the initial energy was 2000 j? 1. write the equation 2.list out your known variables 3.plug the numbers into the equations 4.solve 5.write your solution statement that includes initial energy and final
Answers: 3

Physics, 22.06.2019 12:10
Energy flows from the producer level to the level. is called
Answers: 1

Physics, 22.06.2019 12:20
Which of the following situations is impossible? a) an object has velocity directed east and acceleration directed east. b) an object has zero velocity but non-zero acceleration. c) an object has constant non-zero velocity and changing acceleration. d) an object has velocity directed east and acceleration directed west. e) an object has constant non-zero acceleration and changing velocity.
Answers: 2

Physics, 22.06.2019 15:00
Give an example in which the electrical energy changes to light energy
Answers: 1
You know the right answer?
Question 17 a sample of iron is put into a calorimeter (see sketch at right) that contains of water....
Questions


Mathematics, 07.07.2019 14:30

Biology, 07.07.2019 14:30

History, 07.07.2019 14:30

History, 07.07.2019 14:30


Biology, 07.07.2019 14:30


English, 07.07.2019 14:30


Mathematics, 07.07.2019 14:30

Mathematics, 07.07.2019 14:30

Mathematics, 07.07.2019 14:30

Mathematics, 07.07.2019 14:30

History, 07.07.2019 14:30

Mathematics, 07.07.2019 14:30

History, 07.07.2019 14:30

Mathematics, 07.07.2019 14:30

History, 07.07.2019 14:30

Physics, 07.07.2019 14:40

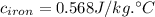
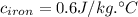 (rounded to 1 decimal place)
(rounded to 1 decimal place)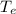 . The energy lost from the iron will be equal to the energy gained by the water. It is assumed that the only heat exchange is between the iron and water and no exchange with the surroundings.
. The energy lost from the iron will be equal to the energy gained by the water. It is assumed that the only heat exchange is between the iron and water and no exchange with the surroundings.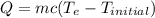 (Eq 1)
(Eq 1)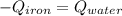 (Eq 2)
(Eq 2)