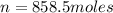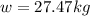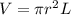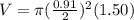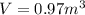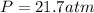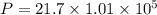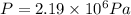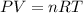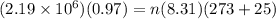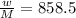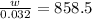An empty cylindrical canister 1.50 m long and 91.0 cm in diameter is to be filled with pure oxygen at 25.0 c to store in a space station. to hold as much gas as possible, the absolute pressure of the oxygen will be 21.7atm . the molar mass of oxygen is 32.0g/mol .finda. how many moles of oxygen does this canister hold? b. for someone lifting this canister, by how many kilograms does this gas increase the mass to be lifted?

Answers: 2
Another question on Physics

Physics, 22.06.2019 03:00
Standing side by side ,you and a friend step off a bridge at different times and fall for 1.6s to the water below.your friend goes first,and you follow after he has dropped a distance of 2.0m.when your friend hits the water,is the seperation between the two of you 2.0m or more than 2.0m? verify your answer with a calculation.
Answers: 2

Physics, 22.06.2019 11:30
Two 1.20-m nonconducting wires meet at a right angle. one segment carries + 2.50 µc of charge distributed uniformly along its length, and the other carries - 2.50 µc distributed uniformly along it, as shown in fig. 21.50. ( a. find the magnitude and direction of the electric field these wires produce at point p, which is 60.0 cm from each wire. ( b. if an electron is released at p, what are the magnitude and direction of the net force that these wires exert on it?
Answers: 3


Physics, 22.06.2019 23:30
Which statement correctly describes the interaction between magnetic poles? north and south poles attract each other. north and south poles repel each other. two north poles will attract each other. two south poles will attract each other.
Answers: 1
You know the right answer?
An empty cylindrical canister 1.50 m long and 91.0 cm in diameter is to be filled with pure oxygen a...
Questions



Mathematics, 13.08.2019 01:20

History, 13.08.2019 01:20

Mathematics, 13.08.2019 01:20

Mathematics, 13.08.2019 01:20


Advanced Placement (AP), 13.08.2019 01:20

Mathematics, 13.08.2019 01:20

Mathematics, 13.08.2019 01:20