
Physics, 07.12.2019 02:31 jesusdelao
Consider the reaction: n2(g) + 3 f2(g) → 2 nf3(g) δh° = -249 kj and δs° = -278 j/k at 25°c calculate δg° and state whether the equilibrium composition should favor reactants or products at standard conditions.

Answers: 2
Another question on Physics

Physics, 22.06.2019 06:00
An ideal gas is held in a container at constant volume. initially, its temperature is 5 degrees c and its pressure is 1.2 atm. what is its pressure when its temperature is 43 degrees c? answer in units of atm
Answers: 2

Physics, 22.06.2019 07:30
The slope of a velocity time graph over any interval of time gives the during that interval
Answers: 2

Physics, 22.06.2019 11:20
How to tell if a molecule is polar or nonpolar with electronegativity
Answers: 2

Physics, 22.06.2019 13:20
This energy transformation diagram represents the energy of a skateboarder moving along a half-pipe. as she skates toward the top of the half-pipe, her original kinetic energy is converted to potential energy and friction. how much of the energy is potential?
Answers: 3
You know the right answer?
Consider the reaction: n2(g) + 3 f2(g) → 2 nf3(g) δh° = -249 kj and δs° = -278 j/k at 25°c calculat...
Questions

History, 30.07.2021 14:00

Business, 30.07.2021 14:00

English, 30.07.2021 14:00

Social Studies, 30.07.2021 14:00

Health, 30.07.2021 14:00



English, 30.07.2021 14:00


Physics, 30.07.2021 14:00


English, 30.07.2021 14:00



Biology, 30.07.2021 14:10

Law, 30.07.2021 14:10




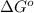 is -166156 J and equilibrium composition should favor products at standard conditions.
is -166156 J and equilibrium composition should favor products at standard conditions.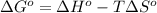
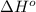 = standard enthalpy = -249 kJ = -249000 J
= standard enthalpy = -249 kJ = -249000 J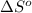 = standard entropy = -278 J/K
= standard entropy = -278 J/K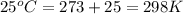

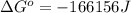
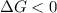 and reaction will be favored in the forward direction that means favored in products.A reaction to be non-spontaneous when
and reaction will be favored in the forward direction that means favored in products.A reaction to be non-spontaneous when 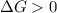 and reaction will be favored in the backward direction that means favored in reactants.
and reaction will be favored in the backward direction that means favored in reactants.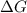 is less than zero that means the reaction is spontaneous and reaction will be favored in the forward direction that means favored in products.
is less than zero that means the reaction is spontaneous and reaction will be favored in the forward direction that means favored in products.

